- Primary care
- Emergency medicine, Resuscitation
- Semi-automatic external defibrillator
- CU Medical Germany GmbH
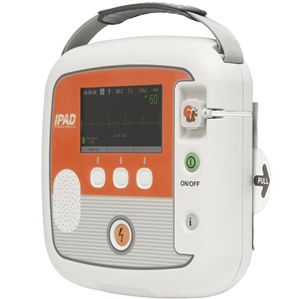
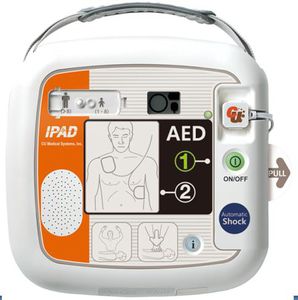
Semi-automatic external defibrillator NF1200public accesswirelesswith ECG monitor
Add to favorites
Compare this product
Characteristics
- Operation
- semi-automatic
- Applications
- public access
- Other characteristics
- biphasic, wireless, with ECG monitor
- Discharge power
200 J
- Number of shocks
200 unit
- Battery power
4.2 Ah
- Weight
2.2 kg
(4.9 lb)- Height
82 mm
(3.2 in)- Length
281 mm
(11.1 in)- Width
220 mm
(8.7 in)
Description
• Automatic Self-testing
• CPR coaching
• Multi event recording
• Pads status détection
• Simple operation
• LEO status indicator
❖ Technical Spécifications
• DEFIBRILLATOR
- Shock-to-Shock Cycle Time : Typically less than 20 seconds
- Protocol : Voice prompts and indicators guide user through protocol.
Follow preconfigured settings. Can be modified with software
- Voice Instructions : Detailed voice messages guide responder through use of the defibrillator
- Controls : Shock Button (NF1200 only), i-Button, On/oft Button
- Indicators : U LEOs Idifferent colors), i-Button
• ENVIRONMENTAL / PHYSICAL REQUIREMENTS
-Temperature : Operating : 32° -110° F10°- A3"CI
Standby : 32° - 110° F |0° - 431!)
- Humidity : Operating - 0% to 60% relative, non-condensing
Standby - 0% to 95% relative, non-condensing
- Vibration : Meets EN1789 random and swept sine, road ambulance spécification
in operating and standby States
- EMI (Radiated/lmmunity) : Meets EN55011 Group 1 Level B Class B and EN61000-4-3
• Sealing
- Meets IEC60529 dass IP54 with battery installed
• AUTOMATED AND USER-ACTIVATED SELF-TESTS
- Daily Self-Tests : Tests internai circuitry, waveform delivery system, battery capacity and software
- Battery Insertion Test : Upon battery insertion, extensive automatic self-tests and user-interactive test check device readiness
Catalogs
No catalogs are available for this product.
See all of CU Medical Germany GmbH‘s catalogsRelated Searches
- External defibrillator
- Portable ventilator
- Emergency ventilator
- Automated external defibrillator
- Semi-automatic external defibrillator
- Biphasic external defibrillator
- External defibrillator with monitor
- Manual external defibrillator
- External defibrillator with ECG monitor
- Training external defibrillator
- Public access external defibrillator
- External defibrillator with multi-parameter monitor
- External defibrillator with touchscreen
- Ventilator with chest compressor
- CPR ventilator
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.