- Secondary care
- Cardiology
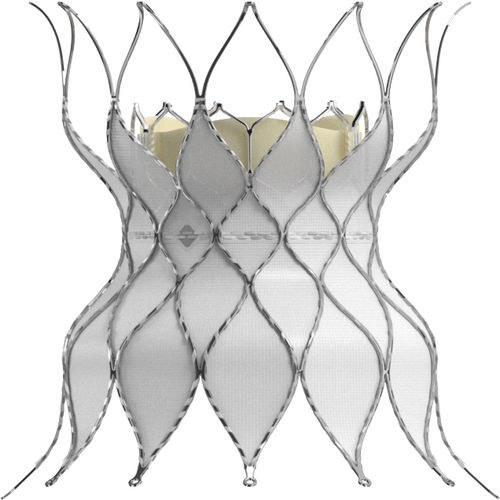
- Pulmonary valve bioprosthesis
- Edwards Lifesciences
Pulmonary valve bioprosthesis SAPIEN 3bovine tissuetitaniumsutureless
Add to favorites
Compare this product
Characteristics
- Valve type
- pulmonary
- Material
- bovine tissue, titanium
- Delivery
- sutureless
- Patient type
- pediatric
Description
Available for the treatment of pediatric and adult patients with dysfunctional RVOT conduit or surgical bioprosthetic valve in the pulmonic position.
Delayed surgical intervention
A less invasive alternative to surgery for patients with a history of pulmonary valve intervention.
100%
Freedom from surgical re-intervention at 1 year*
Freedom from reintervention with transcatheter pulmonic valve replacement (TPVR) or valve-in-valve at 1 year*
Excellent clinical performance
The major risks associated with the transcatheter pulmonic valve procedure include death, heart damage potentially requiring surgery, bleeding, blood vessel complications, and irregular heart beat.
0%
All cause death at 1 year† (n=51)^
Endocarditis at 1 year (n=51)^
Valve frame fracture at 1 year (n=51)^
98.1%
Device success rate (n=53/54)†^
93%
None/trace paravalvular regurgitation at 1 year (n=47)^
^(n=56) COMPASSION S3 trial results using the 20, 23, 26, and 29 mm SAPIEN 3 valve in dysfunctional RVOT conduit or surgical heart valve, valve implant population
† Device success is a composite of
Single THV implanted in the desired location
RV-PA peak-to-peak gradient < 35 mmHg post implantation
Less than moderate PR by discharge TTE (or earliest evaluable TTE)
Free of explant at 24 hours post implantation
Skirt
Polyethylene terephthalate (PET) outer skirt is designed to minimize paravalvular leak
Valve tissue
Utilizes the same bovine pericardium tissue and processes** as Edwards surgical valves
Frame design
Enhanced frame geometry for low profile delivery
Edwards SAPIEN 3 transcatheter pulmonary valve (TPV) system with Alterra adaptive prestent
Catalogs
No catalogs are available for this product.
See all of Edwards Lifesciences‘s catalogsRelated Searches
- Valve bioprosthesis
- Bovine tissue valve bioprosthesis
- Aortic valve bioprosthesis
- Valve prosthesis
- Annuloplasty ring
- Sutureless valve bioprosthesis
- Mitral annuloplasty ring
- Aortic valve prosthesis
- Mitral valve bioprosthesis
- Nitinol valve bioprosthesis
- Mitral valve prosthesis
- Cardiac restoration system
- Mitral valve cardiac restoration system
- Pulmonary valve bioprosthesis
- Tricuspid annuloplasty ring
- Tricuspid valve bioprosthesis
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.



