- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
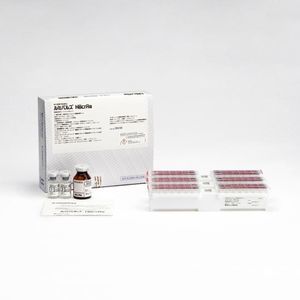
Hepatitis C test kit INNO-LIA®for infectious diseasesfor antigensHCV
Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases, hepatitis C
- Tested parameter
- for antigens
- Micro-organism
- HCV
- Sample type
- serum, plasma
- Analysis mode
- line immunoassay, automated
- Format
- strip
Description
Line immunoassay for the detection of antibodies to human hepatitis C virus in human serum and plasma. It is intended for use as a supplementary test on human serum or plasma specimens found to be reactive in an anti-HCV screening procedure.
The INNO-LIA HCV Score assay utilizes well-defined antigens derived from HCV immunodominant proteins from the core region, the E2 hypervariable region (HVR), the NS3 helicase region and the NS4A, NS4B and NS5A regions. The antigens used are either recombinant proteins or synthetic peptides, highly purified, and fixed on a nylon membrane.
Details
Features & Benefits
Low follow-up rate
Low indeterminate rate
High sensitivity in BBI panels
Short and overnight procedures
Color-coded reagents
Ready-to-use reagents
Four control lines to monitor 1) non-specific reactivity, 2) sample addition, 3) and 4) color development steps
Fully automated strip processing possible using TENDIGO™, Auto-LIA™ 48, AutoBlot 3000(H) or Roboblot®
Objective, automated reading and interpretation of the strips possible using LiRAS® for Infectious Diseases
Related Searches
- Fujirebio test kit
- Fujirebio solution reagent
- Fujirebio blood test kit
- Fujirebio serum test kit
- Fujirebio immunoassay test kit
- Fujirebio plasma test kit
- Research reagent kit
- Fujirebio infectious disease test kit
- Protein reagent kit
- Fujirebio diagnostic reagent
- Fujirebio laboratory reagent
- Enzyme reagent kit
- Fujirebio molecular biology test kit
- Fujirebio respiratory disease test kit
- Whole blood detection kit
- Histology reagent kit
- Optical assay kit
- Fujirebio clinical test kit
- Immunology reagent
- Cassette assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.