- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
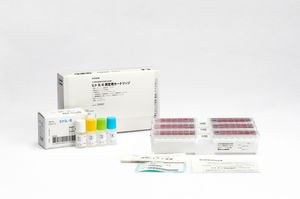
PCT assay kit Lumipulse® G B•R•A•H•M•Ssepsisfor inflammatory diseasesC-reactive protein
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Applications
- sepsis, for inflammatory diseases
- Tested parameter
- C-reactive protein, PCT
- Sample type
- clinical, serum, plasma
- Analysis mode
- enzyme immunoassay, CLEIA
- Result display time
6 h
Description
Immunoreaction cartridges for in vitro diagnostic (IVD) use with a two-step sandwich immunoassay method on the LUMIPULSE G system for the quantitative determination of procalcitonin (PCT) in human serum and plasma.
The assay utilises proven CLEIA (chemiluminescent enzyme immunoassay) technology with results that are available in up to 35 minutes.
Details
Clinical Background
Procalcitonin (PCT) is a biomarker used for the diagnosis of bacterial infection and sepsis. The use of PCT has increased in clinical practice for improved patient management, in a variety of settings including primary care, emergency room (ER) and intensive care units (ICU).
PCT has advantages over other biomarkers for sepsis, such as C-Reactive Protein (CRP) and white blood cell count:
Specificity for bacterial infection (versus inflammation in general).
Rapidity of rise after an insult (6 hrs).
Rapid decline on infection with immune control (half-life of 24 hrs).
Excellent correlation with severity of illness.
Related Searches
- FUJIREBIO test kit
- FUJIREBIO solution reagent
- FUJIREBIO blood test kit
- FUJIREBIO serum test kit
- FUJIREBIO immunoassay test kit
- FUJIREBIO plasma test kit
- FUJIREBIO infectious disease test kit
- FUJIREBIO research reagent
- FUJIREBIO diagnostic reagent
- FUJIREBIO laboratory reagent
- FUJIREBIO protein reagent
- FUJIREBIO molecular biology test kit
- Enzyme reagent kit
- Whole blood detection kit
- FUJIREBIO respiratory disease test kit
- Histology reagent kit
- Optical assay kit
- FUJIREBIO clinical test kit
- Cassette assay kit
- Immunology reagent
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.