- Laboratory
- Laboratory medicine
- Resin reagent
- G-BIOSCIENCES
Resin reagent 786-285for researchproteindensity
Add to favorites
Compare this product
Characteristics
- Type
- resin
- Applications
- for research
- Tested parameter
- protein, density, metal, copper, zinc
Description
Immobilized Metal Ion Affinity Chromatography (IMAC), developed by Porath et al (1), is based on the interaction of certain protein residues (histidines, cysteines, and to some extent tryptophans) with cations of transition metals. The Copper Chelating Resin is specifically designed for the purification of proteins that associate with copper ions (2-3), including 6x histidine tagged proteins.
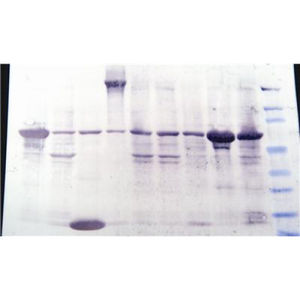
Immobilized metal affinity chromatography (IMAC) resin utilizing copper (Cu2+) for the purification of 6x histidine tagged proteins.
This resin binds to six histidine residues (6x His), a common tag used in protein purification. The resin consists of iminodiacetate coupled to 6% cross-linked agarose beads. The iminodiacetate binds divalent copper ion with a capacity of 20-40μmoles Cu2+/ml resin. The protein binding capacity is >50mg protein per ml resin. We have demonstrated binding of >100mg of a 50kDa 6XHis tagged proteins to a ml of resin.
Immobilized Nickel, Cobalt and Zinc Chelating Resins are also available. Cobalt has the highest selectivity followed by Zinc, Nickel then Copper, but has the lowest loading capacity. Copper has the highest loading capacity, followed by Nickel then Zinc.
Specific binding/wash and elution buffers are available.
Features
For the purification of Copper Binding proteins, including 6x His proteins
High capacity: >50mg/ml
Ligand density: 20-40μmoles Cu2+ /ml resin
Bead Structure: 6% cross-linked agarose
Applications
Affinity purification of copper binding proteins
Affinity purification of proteins with a 6x His tag.
Catalogs
No catalogs are available for this product.
See all of G-BIOSCIENCES‘s catalogsRelated Searches
- Assay kit
- Solution reagent kit
- Molecular biology reagent kit
- Research reagent kit
- Protein reagent kit
- Laboratory reagent kit
- Enzyme reagent kit
- Histology reagent kit
- Optical assay kit
- Reagent medium reagent kit
- Immunology reagent
- Cytology reagent kit
- Dye reagent
- Antibody
- Buffer solution reagent kit
- Clinical chemistry reagent
- Bacteria reagent kit
- Research assay kit
- Virus reagent kit
- Laboratory detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.