- Laboratory
- Sample management
- Clinical collection kit
- GCMedica Enterprise

- Products
- Catalogs
- News & Trends
- Exhibitions
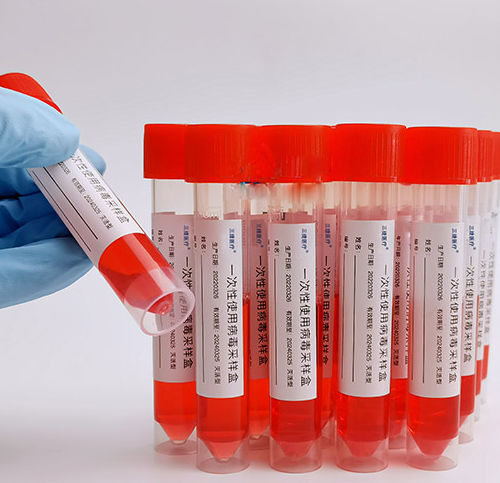
Clinical sampling kit GC4053B0 series
Add to favorites
Compare this product
Characteristics
- Sample type
- clinical
Description
Viral Sampling Kit is designed for the inactivation of a variety of viruses, such as clinical Covid-19, influenza, avian influenza, hand-foot-mouth, measles virus, norovirus, rotavirus and other virus specimens, and for the transportation of the virus nucleic acids.
Features of Virus Sampling Kit
Compatible with various viral nucleic acid extraction kits available on the market and can achieve seamless connection with downstream nucleic acid extraction
Contains an efficient virus lysis solution, which inactivates the virus immediately after sampling, effectively preventing the risk of secondary infection
Storage at room temperature for 48 hours does not affect the quantitative results of nucleic acids. The nucleic acid in positive samples can still be detected after 7 days
Non-inactivated virus preservation solutions are also available.
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.

