- Products
- Catalogs
- News & Trends
- Exhibitions
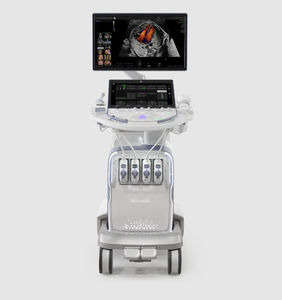
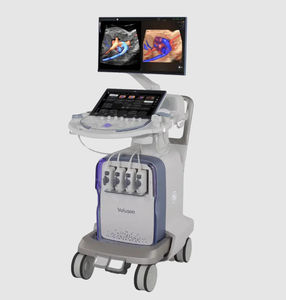
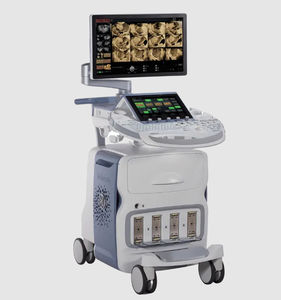
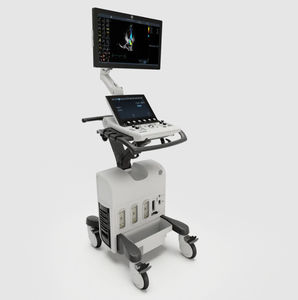
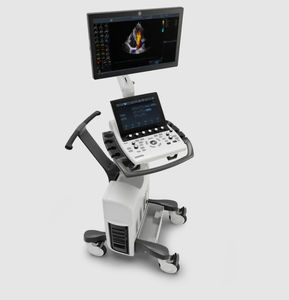
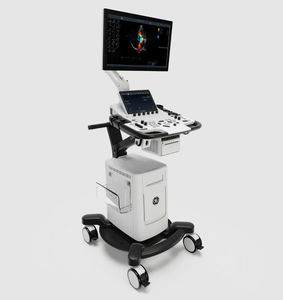
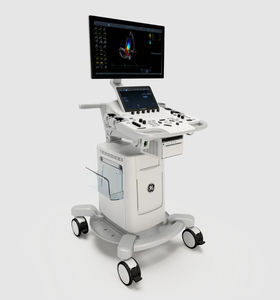
On-platform ultrasound system Invenia™ ABUSfor breast ultrasound imagingB/Welastography

Add to favorites
Compare this product
Characteristics
- Ergonomics
- on-platform
- Application
- for breast ultrasound imaging
- Imaging modes
- B/W, elastography
Description
Invenia ABUS 2.0, is the first FDA-approved ultrasound supplemental screening technology specifically designed for detecting cancer in dense breast tissue.
Designed for screening
Improve breast cancer detection by 35.7 percent over mammography alone1
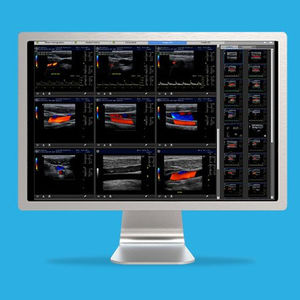
Remarkable image quality
Look differently at dense breast tissue with advanced interpretation tools
AI Assistant
Helps to detect and characterize breast lesions for clinical confidence
Invenia ABUS 2.0 transforms breast care from reactive to proactive, helping to detect breast cancers in dense tissue
Approximately 40% of women have dense breasts2, one of the strongest common risk factors for developing breast cancer3. Clinical evidence is growing about the effectiveness of ultrasound for finding small, node-negative, invasive cancers missed by mammography4. Invenia ABUS 2.0 supplemental imaging is designed for the screening environment, specifically for dense breast imaging.
Personalized breast care with Invenia ABUS 2.0 for early detection, confident diagnosis, and treatment along the breast care pathway.
Dense breast tissue and cancer appear white on a mammogram, potentially camouflaging small cancers. Invenia ABUS 2.0, is specifically designed to help clinicians find cancers that may be hidden on mammography
The power of early cancer detection
Supplemental screening with Invenia ABUS 2.0 transforms breast care from reactive to proactive. When used in addition to mammography, Invenia ABUS 2.0 can improve breast cancer detection by 35.7 percent over mammography alone5. Women whose breast cancer is detected at an early stage have a 90% or higher survival rate
VIDEO
Catalogs
Invenia™ABUS
6 Pages
Related Searches
- GE ultrasound system
- GE B/W ultrasound system
- GE color doppler ultrasound system
- Multipurpose ultrasound imaging system
- Portable ultrasound system
- Convex-array ultrasound system
- Linear-array ultrasound system
- GE on-platform ultrasound system
- Hand-held ultrasound system
- GE touchscreen ultrasound system
- Wireless probe ultrasound system
- Spectral doppler ultrasound system
- Built-in console ultrasound system
- GE 3D/4D ultrasound system
- GE cardiovascular ultrasound imaging system
- Urology ultrasound imaging system
- Endocavitary ultrasound system
- 15-inch ultrasound system
- Elastography ultrasound system
- Breast ultrasound imaging system
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.