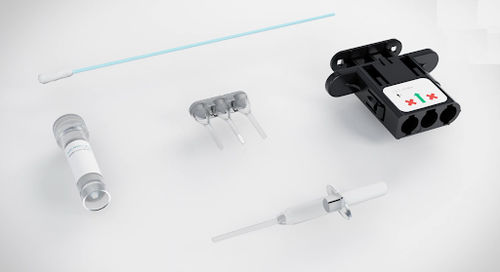
Wound management test kit Genedrive®geneticmolecular

Add to favorites
Compare this product
Characteristics
- Application field
- wound management
- Tested parameter
- genetic
- Analysis mode
- molecular
- Result display time
26 min
- Specificity
100 %
- Sensitivity
100 %
Description
Prevention - reduces the likelihood of aminoglycoside induced hearing loss
Rapid results - point-of-care genetic test yields results in 30 minutes
Simple to use - by healthcare professionals with minimal training
An in vitro diagnostic (IVD) molecular assay for use in human buccal cells
The Genedrive® MT-RNR1 ID Kit used in conjunction with the Genedrive® System provides an automated result of an individual's MT-RNR1 m.1555 variant status to inform the clinician ahead of antibiotic treatment decisions.
The Genedrive® MT-RNR1 ID Kit is intended to be used by healthcare professionals within a near patient setting.
The Genedrive® System is a compact benchtop system that provides rapid nucleic amplification, detection and result reporting without the need for data interpretation
Reduces the likelihood of aminoglycoside induced hearing loss
Provides a clear patient benefit by informing the clinician ahead of prescription
Rapid genetic screening prior to aminoglycoside treatment
Individuals with the MT-RNR1 m.1555A>G variant develop profound irreversible hearing loss if exposed to aminoglycoside - population-based studies estimate prevalence of 1:500 (0.2%)
Single use, cost effective test for use by healthcare professionals with minimal training
Easy adoption into existing neonatal admissions process to inform clinicians ahead of antibiotic treatment decisions
Non-invasive test using buccal swabs from the inner cheek
VIDEO
Catalogs
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.






