- Laboratory
- Laboratory medicine
- Medium reagent
- Guangdong Ardent BioMed Co., Ltd
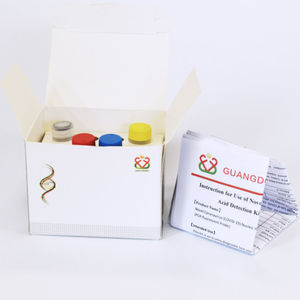
Free extraction viral transport medium reagent buffer solutiondiagnosticfor nucleic acids
Add to favorites
Compare this product
Characteristics
- Type
- free extraction viral transport medium, buffer solution
- Applications
- diagnostic, for nucleic acids
- Micro-organism
- coronavirus, SARS-COV-2
Description
The step of extraction is no longer needed,therefore the procedure of detection has been optimized with shortened time and simplified operation, as the loss of samples during the extraction is also avoided.
The inactivation of preserved viruses ensures the operation of detection is safe.
The compound contains no PCR inhibitors and the preserved samples are convenient for follow-up experiments.
Preprocess the collected samples to release the target matter from the combination with other elements for detection experiment with in vitro diagnostic reagents or equipment.
The compound can release the nucleic acid from samples by lysis and keep the nucleic acid intact and stable. Furthermore, the compound does not inhibit the PCR or other follow-up experiments.
nucleic acid releasing buffer, sample preserving buffer.
Store at room temperature and stable for 12 months.
This product is suitable for processing cells, viruses, and microbes.
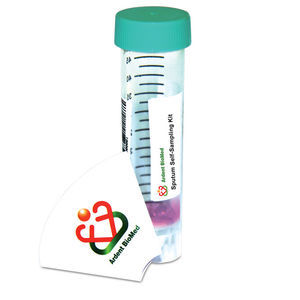
Nasal swabs
Insert the head of swab into the nasal cavity gently and wipe the nasal cavity several times to collect samples. Then insert the head of swab into the buffer and discard the tail of swab.
Throat swabs
Wipe the tonsil and posterior pharyngeal wall with the head of swab to collect samples. Then insert the head of swab into the buffer and discard the tail of swab.
TipS:
The novel coronaviruses belong to the p genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source.
Catalogs
No catalogs are available for this product.
See all of Guangdong Ardent BioMed Co., Ltd‘s catalogsOther Guangdong Ardent BioMed Co., Ltd products
COVID-19
Related Searches
- Solution reagent kit
- Molecular biology reagent kit
- Research reagent kit
- Protein reagent kit
- Diagnostic reagent kit
- Enzyme reagent kit
- Reagent medium reagent kit
- Buffer solution reagent kit
- PCR reagent kit
- Virus reagent kit
- Nucleic acid reagent kit
- NGS reagent kit
- DNA polymerase reagent kit
- DNA purification reagent kit
- RNA extraction reagent kit
- Coronavirus reagent kit
- Antibody reagent kit
- Lipoprotein reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.