- Medical Technical Facilities
- Intensive care
- Hemodialysis catheter
- Guangdong Baihe Medical Technology
- Products
- Catalogs
- News & Trends
- Exhibitions
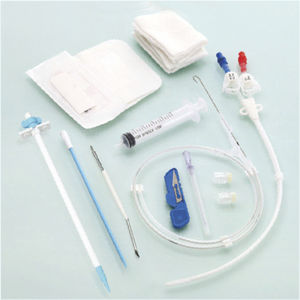
Hemodialysis catheter ABLE®pressure monitoringblood vesselcentral venous
Add to favorites
Compare this product
Characteristics
- Application
- hemodialysis, pressure monitoring
- Area of the body
- blood vessel, central venous
- Number of lumen
- double-lumen
- Outer diameter
- 12 FR
- Total length
160 mm, 200 mm
(6 in, 8 in)
Description
The ABLE® Haemodialysis Catheters (HDC) are sterile, single-use only polyurethane catheters designed to build a temporary blood connection between the patient and the dialysis machine for acute and short-term dialysis. They are available in variety of lumen configurations, lengths, French sizes. The multi lumen variants provide dedicated lumens for haemodialysis therapy, infusion therapy, pressure monitoring. The HDC are packaged along with the components and accessories for insertion with Seldinger technique.All products are sterilized by ethylene oxide.
Intended Use
The ABLE® Haemodialysis Catheters may be applicable to the one of following therapy:
- To supply the temporary blood vessel access in haemodialysis treatment;
- Monitor of central venous pressure;
- Continuous or discontinuous venous transfusion.
The Catheter is surgically penetrated into three optional puncture points depended on the clinical requirement with Seldinger Technique. The Insertion Sites are :
1. Internal jugular vein;
2. Subclavian vein;
3. Femoral vein.
It is possible to be inserted inside the body for no more than 30 days. If duration exceeds 30 days, it may occur the risk of combining the catheter and inside tissue, which result in serious incident.
Contraindications
1. Infection or cut wound around the puncture area.
2. Dysfunction of blood coagulation.
3. During the anticoagulant treatment.
4. Symptoms of inadaptability to puncture operation, such as Pneumothorax, vein sclerosis.
5. Abnormal or unclear anatomical situation at the penetration area, such as sever emphysema, obviously inadaptability from previous operation
Catalogs
Exhibitions
Meet this supplier at the following exhibition(s):

Other Guangdong Baihe Medical Technology products
Blood Purification
Related Searches
- Catheter
- Peripheral catheter
- 6 FR catheter
- Diagnostic catheter
- 12 FR catheter
- 8 FR catheter
- 14 FR catheter
- Venous catheter
- Access catheter
- Double-lumen catheter
- 10 FR catheter
- Membrane filter
- Silicone catheter
- Central venous catheter
- 7 FR catheter
- Blood vessel catheter
- Triple-lumen catheter
- Hemodialysis catheter
- Water filter
- Blood filter
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.