- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- Hangzhou GENESIS Biodetection and Biocontrol CO., LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
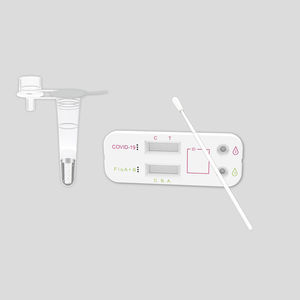
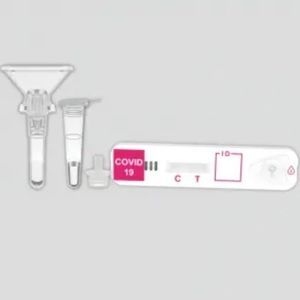
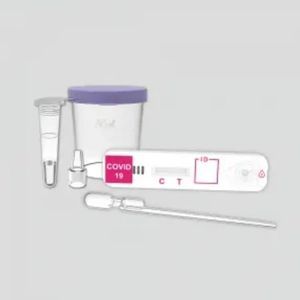
COVID-19 rapid test KaiBiLi™IgGIgMcoronavirus

Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- IgG, IgM
- Micro-organism
- coronavirus
- Sample type
- serum, plasma, whole blood, clinical
- Analysis mode
- lateral flow, chromatographic immunoassay
- Result display time
15 min, 30 min
- Specificity
97 %, 98.5 %
- Sensitivity
82.8 %, 98.3 %
Description
The KaiBiLiTM COVID-19 IgG/IgM Rapid Test Device is a lateral flow chromatographic immunoassay for the qualitative detection of IgG and IgM antibodies to 2019- Novel Coronavirus in human whole blood, serum or plasma specimen. It is only used as a supplementary detection indicator for new suspected cases of negative coronavirus nucleic acid detection or in conjunction with nucleic acid detection in the diagnosis of suspected cases. It cannot be used as a basis for the diagnosis and exclusion of pneumonitis infected by 2019-nCoV infection. It is not suitable for general population screening.
Any reactive specimen with the COVID-19 IgG/IgM Rapid Test Device must be confirmed with alternative testing method(s) and clinical findings. A positive test result requires further confirmation. A negative results do not preclude acute 2019-nCoV infection. If acute infection is suspected, direct testing for COVID-19 antigen is necessary. False positive results for COVID-19 IgG/IgM Rapid Test may occur due to cross-reactivity from preexisting antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using second, different IgG or IgM assay.
Detection
The COVID-19 IgG/IgM Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative lateral flow immunechromatographic assay for the detection of IgG and IgM antibodies to 2019-nCoV in whole blood, serum or plasma specimen.
Kit Storage Conditions
2~30°C.
VIDEO
Other Hangzhou GENESIS Biodetection and Biocontrol CO., LTD products
IMMUNOASSAYS
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Reagent medium reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Buffer solution reagent kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.