- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- Hangzhou GENESIS Biodetection and Biocontrol CO., LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
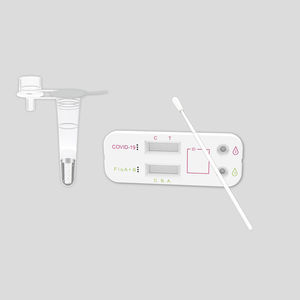
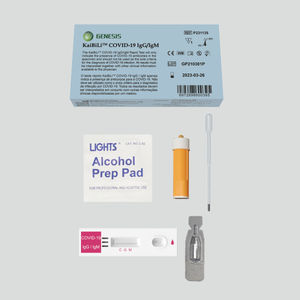
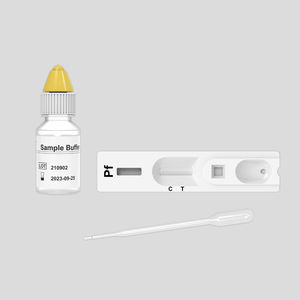
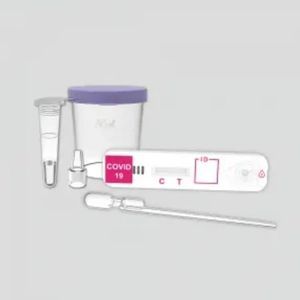
COVID-19 rapid test KaiBiLi ™for antigensproteinSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens, protein
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- nasal, saliva, laboratory, throat
- Analysis mode
- immunochromatographic
- Result display time
15 min, 30 min
Description
The KaiBiLiTM COVID-19 Antigen Saliva Test is an in vitro diagnostic test based on the principle of immuno-chromatography for the qualitative detection of 2019 Novel Coronavirus nucleocapsid protein antigens in saliva.
Introduction
COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main symptoms including manifestations include fever, fatigue and dry cough, nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
The KaiBiLiTM COVID-19 Antigen test device is an immunochromatographic assay for the qualitative detection of 2019 Novel Coronavirus antigens. This assay is intended for rapid screening in laboratory. This test should be conducted by trained technician, wearing appropriate personal protective equipment (PPE). The detection is based on the antibodies which were developed specifically recognizing and reacting with the nucleoprotein of 2019 Novel Coronavirus. It is intended to aid in the rapid diagnosis of SARS-CoV-2 infection.
Detection
For the qualitative detection of 2019 Novel Coronavirus nucleocapsid protein antigens in saliva.
Limit Of Detection (LoD)
140 TCID50/mL.
Accuracy
Positive Percent Agreement:94.8%
Negative Percent Agreement:98.7%
Overall Percent Agreement:97.5%
Kit Storage Conditions
2~30°C.
Other Hangzhou GENESIS Biodetection and Biocontrol CO., LTD products
IMMUNOASSAYS
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Reagent medium reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Buffer solution reagent kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.