- Laboratory
- Laboratory medicine
- Rapid flu test
- Hangzhou GENESIS Biodetection and Biocontrol CO., LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
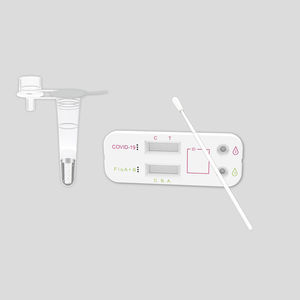
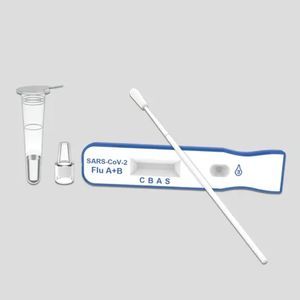
Rapid flu test KaiBiLi™for antigensinfluenza Ainfluenza B
Add to favorites
Compare this product
Characteristics
- Applications
- flu
- Tested parameter
- for antigens
- Micro-organism
- influenza A, influenza B
- Sample type
- nasopharyngeal, nasal, cell
- Analysis mode
- immunochromatographic
- Result display time
15 min, 30 min
- Specificity
97.6 %, 99 %
- Sensitivity
86.7 %, 88.9 %
Description
The KaiBiLiTM Flu A&B Antigen Rapid Test is an in vitro diagnostic test for the qualitative detection of influenza A and B nucleoprotein antigens in nasopharyngeal (NP) swab and nasal aspirate samples, using the rapid immunochromatographic method.
Introduction
The KaiBiLiTM Flu A&B Antigen Rapid Test is an immune - chromatographic membrane assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens in nasopharyngeal (NP) swab, and nasal aspirate samples.
The detection is based on the monoclonal antibodies specific for the nucleoprotein of either influenza virus A or B. It is intended to aid in the rapid diagnosis of influenza A and B viral infection. Negative results should be confirmed by other methods, such as cell culture.
Influenza viruses are causative agents of highly contagious, acute, viral infections of the respiratory tract. Influenza viruses are immunologically diverse, singlestranded RNA viruses, Influenza illness classically presents with sudden onset of fever, chills, headache, myalgias, and a nonproductive cough.
Detection
Detection of influenza A and B nucleoprotein antigens in nasopharyngeal (NP) swab and nasal aspirate samples
Limit Of Detection (LoD)
The limit of detection (LOD) for the KaiBiLiTM Flu A&B Antigen Rapid Test was established for a total of 10 influenza strains: 8 influenza A and 2 influenza B.
Kit Storage Conditions
2~30°C.
Contents
Description
Qty
Flu A&B test devices -
20
Sterilized swabs -
20
Extraction buffer, 7mL -
2
Nozzles with filter -
20
Plastic tubes -
20
Tube Stand -
1
Other Hangzhou GENESIS Biodetection and Biocontrol CO., LTD products
IMMUNOASSAYS
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Rapid lateral flow test
- Laboratory reagent kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Reagent medium reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Buffer solution reagent kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.