- Laboratory
- Laboratory medicine
- Rapid infectious disease test
- Hangzhou GENESIS Biodetection and Biocontrol CO., LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
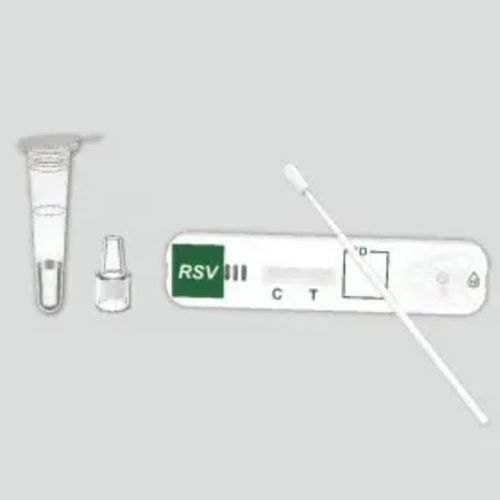
Rapid infectious disease test KaiBiLi™for antigensvirusinfluenza A
Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases
- Tested parameter
- for antigens
- Micro-organism
- virus, influenza A, respiratory syncytial virus, RSV
- Sample type
- nasopharyngeal, nasal, clinical
- Analysis mode
- immunochromatographic
- Result display time
15 min, 30 min
- Specificity
96.8 %
- Sensitivity
91.5 %
Description
The KaiBiLiTM RSV Antigen Rapid Test is an in vitro diagnostic test for the qualitative detection of Respiratory Syncytial Virus (RSV) nucleoprotein antigens in nasopharyngeal (NP) swab and nasal aspirate samples, using the rapid immunochromatographic method.
RSV is a causative agent of highly contagious, acute, viral infection of the respiratory tract in pediatric and elderly populations. Respiratory syncytial virus is a single-stranded RNA virus. Nearly half of all children become infected with RSV in their first year of life. It is also the major viral cause of nosocomial illness in children already hospitalized for other reasons. Worldwide, it is estimated that RSV is responsible for greater than 30 million cases of LRI in children under 5 years of age each year.
Diagnostic methods for detection of respiratory viruses include viral cell culture, direct fluorescent antibody (DFA), rapid immunoassays, and nucleic acid amplification assays such as the polymerase chain reaction (PCR). Each has been demonstrated to have clinical utility for the detection of respiratory viruses including RSV.
Rapid immunoassays available for specific viruses such as influenza A/B and RSV allow a quick diagnosis so that patients may be appropriately isolated and treated to prevent the nosocomial spread of infections to fellow patients with compromised cardiac, respiratory or immune functions.
Detection
Diagnostic test for the qualitative detection of Respiratory Syncytial Virus (RSV) nucleoprotein antigens in nasopharyngeal (NP) swab and nasal aspirate samples,
Limit Of Detection (LoD)
Subtype
Strain
TCID50/mL
A
RSV(long)
32.5
B
RSV(wild-type)
4×102
Kit Storage Conditions
2~30°C.
Other Hangzhou GENESIS Biodetection and Biocontrol CO., LTD products
IMMUNOASSAYS
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Reagent medium reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Buffer solution reagent kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.