- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- Hangzhou GENESIS Biodetection and Biocontrol CO., LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
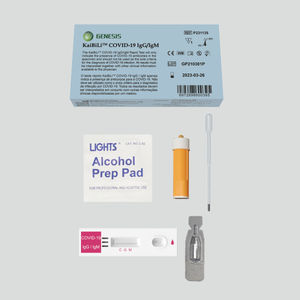
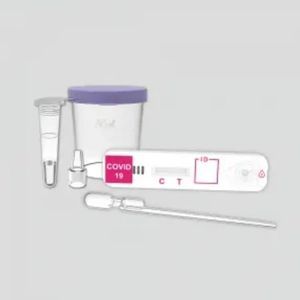
Rapid antibody test KaiBiLi™COVID-19coronavirusblood

Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antibodies
- Micro-organism
- coronavirus
- Sample type
- blood, serum, plasma, whole blood
- Analysis mode
- immunochromatographic, lateral flow, chromatographic immunoassay
- Result display time
15 min, 30 min
- Specificity
100 %
- Sensitivity
91.8 %
Description
The COVID-19 Neutralization Antibody Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative lateral flow immunochromatographic assay for the detection of neutralization antibody to 2019-nCoV in whole blood, serum or plasma.
Introduction
The KaiBiLiTM COVID-19 Neutralization Antibody Rapid Test Device is a lateral flow chromatographic immunoassay for the qualitative detection of neutralization antibody to 2019-Novel Coronavirus in human whole blood, serum or plasma specimen. It is only used as a referential detection indicator for the effect of vaccine. It is not suitable for general population screening. The novel coronaviruses belong to the β genus.
COVID-19 is an acute respiratory infectious disease. 2019-nCOV has several structural proteins including spike (S), envelope (E), membrane (M) and nucleocapsid (N). The spike protein (S) contains a receptor binding domain (RBD), which is responsible for recognizing the cell surface receptor, angiotensin converting enzyme-2 (ACE2). It is found that the RBD of the 2019-nCOV S protein strongly interacts with the human ACE2 receptor leading to endocytosis into the host cells of the deep lung and viral replication. Infection with the 2019-nCOV initiates an immune response, which includes the production of antibodies in the blood. The secreted antibodies provide protection against future infections from viruses, because they remain in the circulatory system for months to years after infection and will bind quickly and strongly to the pathogen to block cellular infiltration and replication. These antibodies are named neutralization antibodies.
VIDEO
Other Hangzhou GENESIS Biodetection and Biocontrol CO., LTD products
IMMUNOASSAYS
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Reagent medium reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Buffer solution reagent kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.