- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- Hangzhou GENESIS Biodetection and Biocontrol CO., LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
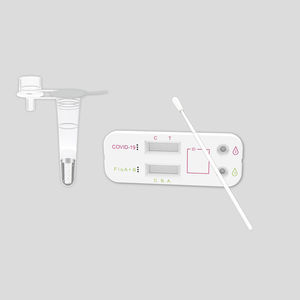
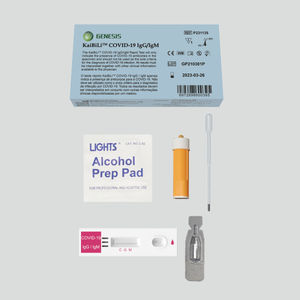
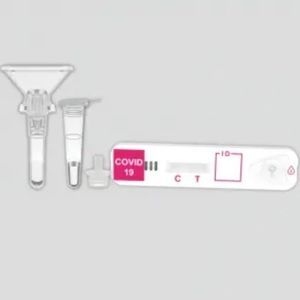
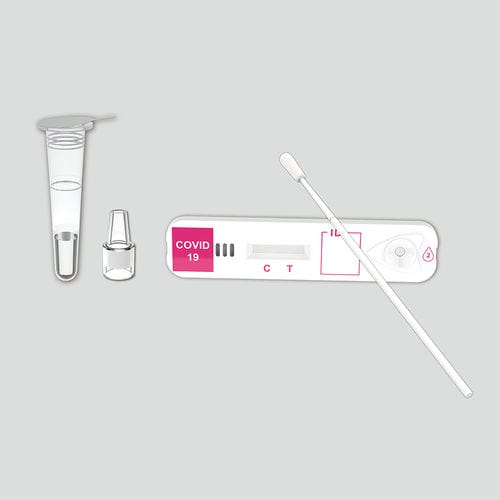
COVID-19 rapid test KaiBiLi™for antigensproteinSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens, protein
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- nasal, saliva, throat
- Result display time
15 min, 30 min
Description
This COVID-19 Antigen - Saliva Swab Rapid Test Device is designed for testing freshly collected saliva samples, for the qualitative detection of 2019 Novel Coronavirus nucleocapsid protein antigens in saliva.
Introduction
SARS-CoV-2 antigen rapid diagnostic tests (Ag-RDT) are being used globally to test suspect COVID-19 cases in contexts where PCR diagnostic may not be available, often at subnational level.
COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main symptoms including manifestations include fever, fatigue and dry cough, nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Individual factors can contribute to SARS-CoV-2 transmission
● People infected with SARS-CoV-2 can transmit virus with or without symptoms
● Most infected people transmit the virus around 2 days before they develop symptoms and up to 9-10 days after symptom onset
● People who develop severe disease can be infectious for longer
● Immunocompromised people can shed the virus for months
● Symptomatic persons infected with SARS-CoV-2 are 3 to 18 times more likely to transmit the virus than asymptomatic persons.
Detection
The qualitative detection of 2019 Novel Coronavirus nucleocapsid protein antigens in saliva.
Limit Of Detection (LoD)
SARS-CoV-2:140TCID50/mL
Accuracy
Positive Percent Agreement: 96.5%
Other Hangzhou GENESIS Biodetection and Biocontrol CO., LTD products
IMMUNOASSAYS
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Rapid lateral flow test
- Laboratory reagent kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Reagent medium reagent kit
- Whole blood rapid diagnostic test
- Buffer solution reagent kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.