- Laboratory
- Laboratory medicine
- Rapid malaria test
- Hangzhou GENESIS Biodetection and Biocontrol CO., LTD

- Products
- Catalogs
- News & Trends
- Exhibitions
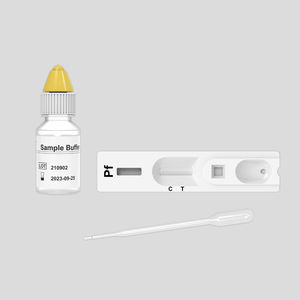
Rapid malaria test P231108for antigensPlasmodiumblood
Add to favorites
Compare this product
Characteristics
- Applications
- malaria
- Tested parameter
- for antigens
- Micro-organism
- Plasmodium
- Sample type
- blood, whole blood, clinical
- Analysis mode
- lateral flow, chromatographic immunoassay
- Result display time
10 min, 20 min
- Specificity
100 %
- Sensitivity
95.9 %, 97.6 %
Description
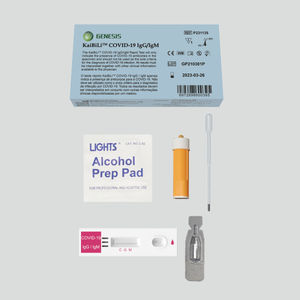
The KaiBiLiTM Malaria Pf/Pv Antigen Rapid Test is a lateral flow chromatographic immunoassay for the qualitative detection of P. falciparum and P. vivax in human whole blood. It is intended to be used as a screening test and aid in the diagnosis of infection with plasmodium. Any reactive specimen with the Malaria Pf/Pv Antigen Rapid Test must be confirmed with alternative testing method(s) and clinical findings. It is not suitable for general population screening.
Introduction
Malaria is an acute mosquito-borne infectious disease caused by the infection of plasmodium, which invades human red blood cells and seriously endangers the health of human beings. It is characterized by fever with chills, anemia and is caused by Plasmodium parasite that is transmitted from one human being to another by the bite of infected Anopheles mosquitoes.
There are four kinds of parasite that can infect humans: P. falciparum, P. vivax, P. ovale, and P. malariae. Of these, P. falciparum causes more severe disease than the other plasmodial species and accounts for most malaria deaths. P. falciparum and P. vivax are the most common pathogens. The disease exists in more than 90 countries worldwide, and it is estimated that there are over 500 million clinical cases and 2.7 million malaria-caused deaths per year.
Detection
The KaiBiLiTM Malaria Pf/Pv Antigen Rapid Test is developed to simultaneously detect and differentiate infection with P. falciparum and P. vivax.
Limit Of Detection (LoD)
Type - Parasites/μL
P. falciparum - 100
P. vivax - 100
Kit Storage Conditions
2~30°C.
Contents
Description - Qty
Test device - 20
Plastic dropper - 20
Sample buffer - 1
Other Hangzhou GENESIS Biodetection and Biocontrol CO., LTD products
IMMUNOASSAYS
Related Searches
- Assay kit
- Solution reagent kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Reagent medium reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Buffer solution reagent kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.