- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- HangZhou Realy Tech co.,Ltd

- Products
- Catalogs
- News & Trends
- Exhibitions
COVID-19 rapid test K511416Dfor antigensSARS-COV-2coronavirus
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antigens
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- sputum, clinical, laboratory, throat
- Format
- cassette
- Result display time
Max.: 20 min
Min.: 10 min
- Specificity
99.9 %
- Sensitivity
96.2 %
Description
The Novel Coronavirus (SARS-Cov-2) Antigen Rapid Test Cassette (swab) is used for the in vitro qualitative detection of novel coronavirus in the throat swabs, sputum samples of suspected pneumonia patients infected by novel coronavirus, suspected clustering cases and others needing diagnosis or differential diagnosis for novel coronavirus.
The definitions of “suspected cases” and “patients with suspected aggregated cases” and other groups are implemented with reference to the “Diagnosis and Treatment Plan for Pneumonia Infected in novel coronavirus” and “Monitoring Plan for Pneumonia Infected in novel coronavirus” and other documents (current version) issued by CDC.
The product is only used for auxiliary diagnosis of related cases and emergency reserve for in vitro diagnosis of this epidemic during the pneumonia epidemic infected by novel coronavirus (SRAS-Cov-2) since December 2019 and it cannot be used as routine in vitro diagnostic reagents in clinical practice. The kit shall comply with the relevant requirements of the “Diagnosis and Treatment Plan for Pneumonia Infected in novel coronavirus” and “Prevention and Control Plan for Pneumonia Infected in novel coronavirus” and other documents in use.
The detection results of this kit are for clinical reference only and should not be used as the sole criteria for clinical diagnosis. It is recommended to conduct a comprehensive analysis on the condition in combination with the clinical manifestations and other laboratory tests.
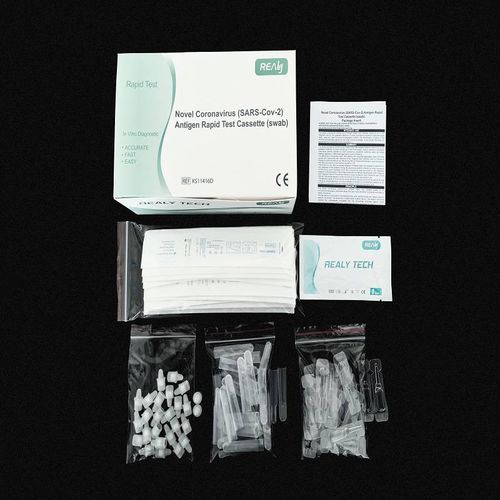
【COMPONENTS OF THE TEST KIT】
● Test Devices
● Sterilized Swab
● Extraction Tube
● Tube Stand
● Nozzle With Filter
● Sample Extraction Buffer
● Package Insert
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.
