- Laboratory
- Laboratory medicine
- Rapid colon cancer test
- Healgen Scientific

- Products
- Catalogs
- News & Trends
- Exhibitions
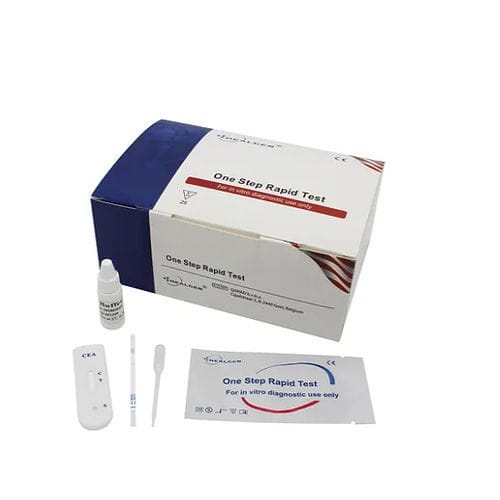
Rapid CEA test GECEA seriescolorectal canceroncologyblood
Add to favorites
Compare this product
Characteristics
- Applications
- colorectal cancer
- Application field
- oncology
- Tested parameter
- CEA
- Sample type
- blood, serum, plasma, whole blood
- Analysis mode
- chromatographic immunoassay
- Format
- cassette, strip
- Result display time
1 min
- Specificity
98.6 %
- Sensitivity
98.5 %
Description
The CEA Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of CEA in whole blood, serum or plasma. It is intended as an aid in the assessment and diagnosis of colorectal cancer and other types of cancer, such as pancreatic, stomach, breast, lung and certain types of thyroid and ovarian cancer. The CEA rapid test device utilizes a combination of colloidal gold conjugate and monoclonal antibodies to selectively detect elevated levels of CEA.
CEA is present in high concentrations during fetal development, but ceases after birth. Therefore, it is not usually present in the blood of healthy adults. The normal CEA range for a non-smoking adult is less than 2.5 ng/mL and less than 5.0 ng/mL in a smoker.
Features
Two band results for simple interpretation
Detects carcinoembryonic antigen
Room temperature storage or refrigerated (2-30⁰C)
Internal control included
Reagents included
Specifications
Cut-off: 5 ng/mL
Shelf Life: 24 months from the date of manufacture
Related Searches
- Assay kit
- Immunoassay assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Virus rapid diagnostic test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Optical assay kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Fluorescence assay kit
- Lateral flow test kit
- Rapid respiratory infection test
- COVID-19 detection kit
- Urine rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.