- Laboratory
- Laboratory medicine
- COVID-19 test kit
- hecin-scientific

- Products
- Catalogs
- News & Trends
- Exhibitions
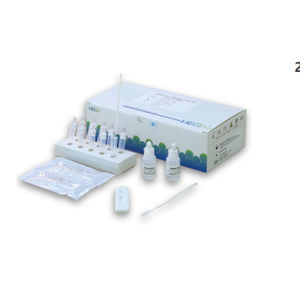
COVID-19 test kit IgGIgMSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- IgG, IgM
- Micro-organism
- SARS-COV-2, coronavirus, adenovirus
- Sample type
- serum, plasma
Description
General name: COVID-19 IgM Antibody Test Kit (colloidal gold method)
[PACKAGE SPECIFICATION]
1 Test/Kit; 20 Tests/Kit; 50 Tests/Kit.
Home ꄲ Product Details-temporary
COVID-19 IgM Antibody Test Kit (colloidal gold method) Instruction
Novel coronavirusIgM antibody in blood was detected in 15 minutes
ꄴ上一个: COVID-19 IgG Antibody Test Kit (colloidal gold method)
ꄲ下一个: Adenovirus nucleic acid detection kit(PCR-Fluorescent probe method)
[PRODUCT NAME]
General name: COVID-19 IgM Antibody Test Kit (colloidal gold method)
[PACKAGE SPECIFICATION]
1 Test/Kit; 20 Tests/Kit; 50 Tests/Kit.
[INTENDED USE]
This kit is only used for qualitative test of SARS-CoV-2 IgM from human serum and plasma in vitro. The kit
is to be used as supplementary test for the SARS-CoV-2-suspected patients whose nuclei acid is tested as
negative, or to be used together with nuclei acid test in the diagnosis of 2019-nCoV-suspected patients. The
test result cannot be used as the sole basis for diagnosis and exclusion decision. The kit is not suitable for the
general population screening.
The kit is provided use only for medical institute.
Positive test result needs to be further confirmed, negative result does not preclude 2019-nCoV infection.
This kit is an emergency clinical use and storage only during COVID-19 pandemics, cannot be used as regular
clinical IVD kit. The test results by this kit are for clinical reference only. It is suggested to consider patient’s
symptoms and other lab tests results to diagnose. Lab test of 2019-nCoV must follow the requirements of
Technical Guidelines for COVID-19 Laboratory Testing and make sure to be equipped with biosafety.
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Protein reagent kit
- Diagnostic reagent kit
- Molecular test kit
- Respiratory infection test kit
- Optical assay kit
- Reagent medium reagent kit
- Cassette assay kit
- Fluorescence assay kit
- Lateral flow test kit
- COVID-19 detection kit
- Antigen assay kit
- Clinical reagent kit
- IgG test kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.