- Primary care
- Emergency medicine, Resuscitation
- Intubation kit
- Henan Province Jianqi Medical Equipment

- Products
- Catalogs
- News & Trends
- Exhibitions
Emergency kit 20152660194intubationdisposablesterile
Add to favorites
Compare this product
Characteristics
- Applications
- emergency, intubation
- Other characteristics
- disposable, sterile
Description
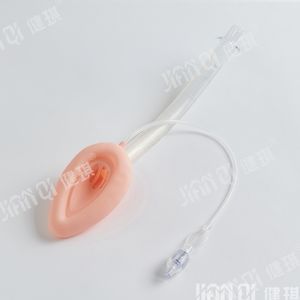
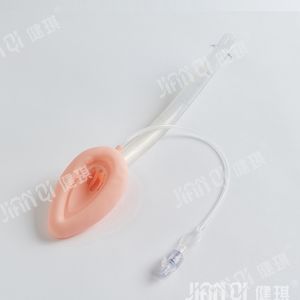
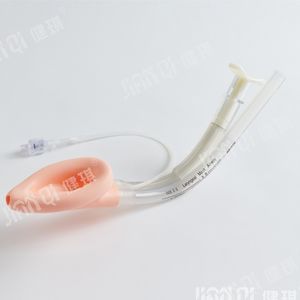
[product name] disposable endotracheal tube assembly
[specification and model] type I (ordinary type) and type II (reinforced type)
[production license] ysyjx production license No. 20080036
[Registration Certificate No yxzz 20152660194
[technical requirements No yxzz 20152660194
[structure and composition] this product consists of basic configuration and optional configuration. Type I basic configuration: endotracheal intubation, medical dental pad, disposable sputum suction tube, disposable suction connecting tube, mouth pad, disposable oropharyngeal airway, medical degreasing gauze block; type II basic configuration: reinforced endotracheal tube, medical dental pad, disposable sputum suction tube, disposable suction connecting tube, mouth pad, disposable oropharyngeal airway, medical degreasing Gauze block; selection and configuration of type I and type II: disposable medical rubber inspection gloves, self-adhesive wound protection patch, medical infusion paste, disposable laryngoscope, disposable sterile rubber surgical gloves, disposable dispensing syringe, endotracheal intubation fixator, medical paraffin cotton ball, wrapping cloth and tray. The product should be sterile. After sterilization with ethylene oxide, the residual amount of ethylene oxide should not be more than 10 μ g / g. Disposable use
[scope of application] it is used to establish artificial airway during clinical anesthesia or emergency.
Exhibitions
Meet this supplier at the following exhibition(s):

Other Henan Province Jianqi Medical Equipment products
Medical polymers
Related Searches
- Safety mask
- Medical kit
- Half-mask safety mask
- Unisex safety mask
- Emergency kit
- White safety mask
- Disposable safety mask
- Non-woven safety mask
- Fabric safety mask
- Endotracheal tube
- Silicone medical mask
- Cup shaped safety mask
- Sterile medical kit
- Supraglottic airway device
- Human laryngeal mask
- Disposable medical kit
- Diagnostic medical kit
- Oral endotracheal tube
- Silicone laryngeal mask
- Surgery medical kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.