- Secondary care
- Gastroenterology
- Bite block
- Henan Province Jianqi Medical Equipment

- Products
- Catalogs
- News & Trends
- Exhibitions
Bite block 20182140534
Add to favorites
Compare this product
Description
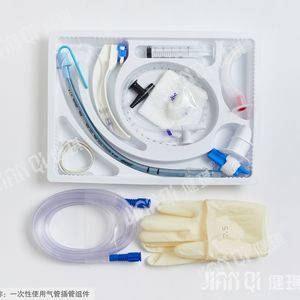
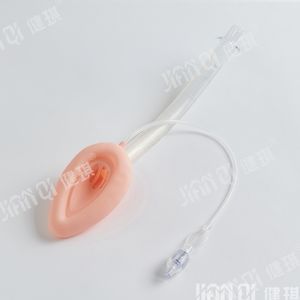
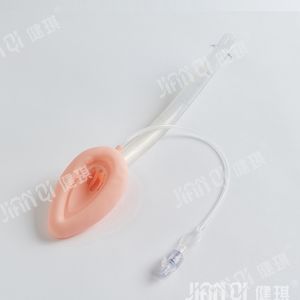
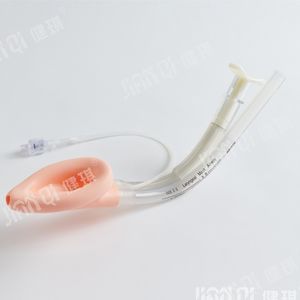
[product name] medical bite block
[model and specification] type I and type II
[product registration certificate No yxzz 20182140534
Performance and structure] the product is composed of tooth pad body and bandage.
[scope of application] it is mainly used for gastroscope operation, examination and intubation.
[usage] open the package and use it according to the routine medical operation.
[precautions]
1) This product is sterilized by ethylene oxide, and the sterilization period is two years.
2) This product is only for one-time use and destroyed after use.
3) Please check the packing before use. If the package is damaged, it is forbidden to use it.
4) See the package seal or label in the bag for the production batch number, sterilization batch number and sterilization validity period.
5) It should be stored in a cool, dry, well ventilated and clean warehouse with relative humidity no more than 80%.
Other Henan Province Jianqi Medical Equipment products
Medical polymers
Related Searches
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.