- Primary care
- Emergency medicine, Resuscitation
- Urinary catheterization medical kit
- Henan Province Jianqi Medical Equipment

- Products
- Catalogs
- News & Trends
- Exhibitions
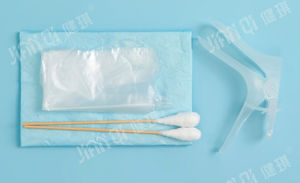
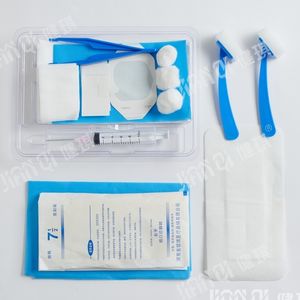
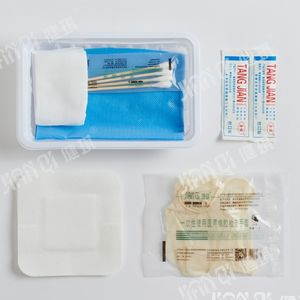
Urinary catheterization medical kit 20172660081disposablesterile
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Applications
- urinary catheterization
- Other characteristics
- disposable, sterile
Description
[product name] disposable sterile catheterization kit
[specification and model] model: single cavity, double chamber, three cavity; specification: fr6, fr8, fr10, fr12, fr14, fr16, FR18, fr20, fr22, fr24, fr26, fr28, FR30;
[production license] ysyjx production license No. 20080036
[Registration Certificate No yxzz 20172660081
[technical requirement No yxzz 20172660081
[structure and composition] this product consists of basic configuration and optional configuration. Basic configuration: disposable sterile catheter, disposable drainage bag, medical tweezers, medical cotton ball and optional configuration: disposable medical rubber inspection gloves, iodophor cotton ball, paraffin cotton ball, medical degreasing gauze block, disposable hole towel, goods box, cloth, sterile water test tube, disposable dispensing syringe, sponge brush, disposable sterilization Rubber surgical gloves and PE inspection gloves.
[product performance] 1. The package of catheterization bag should be well sealed without cracks and holes; the accessories in the bag should be free from damage, stains and impurities. 2. The body, tip, balloon and eyelet of disposable sterile catheter should be free of foreign substances; the nominal outer diameter of catheter should be 0.01mm, and the tolerance should be ± 0.33mm
Other Henan Province Jianqi Medical Equipment products
Medical bag
Related Searches
- Medical kit
- Safety mask
- Emergency kit
- Unisex safety mask
- White safety mask
- Half-mask safety mask
- Disposable safety mask
- Non-woven safety mask
- Sterile medical kit
- Endotracheal tube
- Silicone medical mask
- Cup shaped safety mask
- Fabric safety mask
- Supraglottic airway device
- Disposable medical kit
- Oral endotracheal tube
- Diagnostic medical kit
- Surgery medical kit
- Silicone laryngeal mask
- Disposable laryngeal mask
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.