- Laboratory
- Laboratory medicine
- COVID-19 rapid test
- Henan UDX Biotechnology Co.,Ltd
COVID-19 rapid test for antibodiesSARS-COV-2serum
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- for antibodies
- Micro-organism
- SARS-COV-2
- Sample type
- serum, plasma, whole blood
- Analysis mode
- immunoassay, lateral flow
- Result display time
Min.: 10 min
Max.: 20 min
- Specificity
99.1 %
- Sensitivity
98.6 %
Description
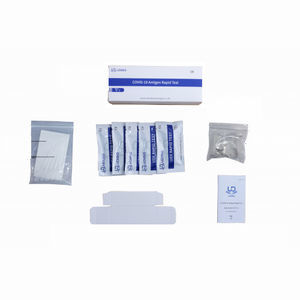
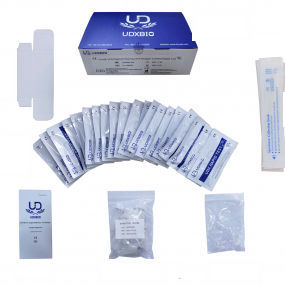
The COVID-19 Total Antibodies Rapid Test is a lateral flow immunoassay intended for the qualitative detection of total antibodies to SARS-CoV-2 in human serum, plasma or whole blood.
The COVID-19 Total Antibodies Rapid test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection.
The product is intended for in vitro detection of SARS-Cov-2 total antibodies in human serum, plasma or whole blood samples.
Parameter of COVID-19 Total Antibodies Rapid Test
Material - pp
Test type - Total Antibodies
Test method - Colloidal Gold
Specimen - Serum, plasma or whole blood
Keep temperature - 2-30℃
Certification - CE, ISO 13485:2016
Shelf-life - 18 months
Package - Carton Box, 1 pc/box, 5 pcs/box, 20 pcs/box,50 pcs/box
Simple. No instrumentation required&simple procedure
Accuracy. Highly sensitivity specificity and accuracy
Convenien. Suitable for large-scale use
Test Principle
This reagent is based on a lateral flow immunoassay.
During the test, specimens are applied to the test cartridges. If SARS-CoV-2 antibody is present in the specimen, they combined with colloidal gold-labeled SARS-CoV-2 recombinant antigen forming antibody-virus antigen-colloidal gold complex (complex T).
During lateral flow, the complex T move along nitrocellulose membrane toward one end of the absorbent paper.
When passing through the line T (coated with same SARS-CoV-2 recombinant antigen), the complex T is captured by recombinant antigen resulting in coloring on line T;
when passing through the line C, colloidal gold-labeled DNP is captured by quality-control antibody resulting in coloring on line C.
Catalogs
No catalogs are available for this product.
See all of Henan UDX Biotechnology Co.,Ltd‘s catalogsOther Henan UDX Biotechnology Co.,Ltd products
COVID-19 RAPID TEST
Related Searches
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Rapid virus test
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Whole blood rapid diagnostic test
- Rapid respiratory infection test
- COVID-19 rapid diagnostic test
- Nasal rapid diagnostic test
- Nasopharyngeal rapid diagnostic test
- Influenza A rapid diagnostic test
- Influenza B rapid diagnostic test
- Electrolyte rapid test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.