
- Products
- Catalogs
- News & Trends
- Exhibitions
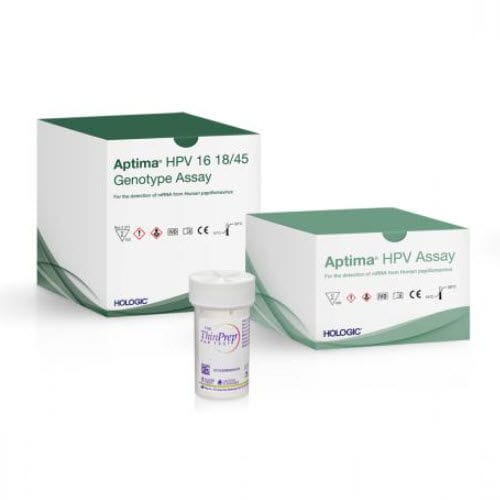
Gynecology test kit Aptima®+ThinPrep®HPVcell
Add to favorites
Compare this product
Characteristics
- Application field
- gynecology
- Micro-organism
- HPV
- Sample type
- cell
Description
Hologic has remained an unwavering advocate for women’s health for more than two decades. Our goals as a company are intrinsically tied to changes in best-practices for women’s health, applying the latest findings in diagnostic medicine to the development of new products and technologies in response to the emergence of new discoveries in medicine.
Cervical disease screening is an essential component of our efforts in women’s health. Hologic is the leader in Pap and human papillomavirus (HPV) testing. The ThinPrep Pap test helps healthcare providers detect the presence of abnormal cervical cells, and the Aptima HPV assays identify high-risk HPV mRNA that is indicative of the HPV infections most likely to lead to cervical disease.
The Pap test has been the most successful cancer screening program in history.4
The rate of cervical cancer, which was a leading cause of death among women, has fallen by more than 70 percent since the Pap test was introduced over 80 years ago.5 Previously, cervical cancer was the leading cause of cancer death in women, but now it is the fifteenth most frequent.
Reducing cervical cancer incidence requires a comprehensive strategy.
Such a strategy will include screening options that take into account disparities that underserved women face. This strategy should focus on reducing preventive screening gaps by:
Implementing provider and patient education
Providing access to vaccinations
Offering Pap + HPV (co-testing)
ACOG, ASCCP, SGO and USPSTF guidelines recommend:
For women ages
21-29 years
Screening with cervical cytology alone every 3 years is recommended.
Catalogs
No catalogs are available for this product.
See all of Hologic‘s catalogsRelated Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Plasma assay kit
- Infectious disease detection kit
- Molecular test kit
- Respiratory infection test kit
- Rapid virus test
- Clinical assay kit
- COVID-19 assay kit
- Clinical chemistry analyzer
- Automatic clinical chemistry analyzer
- Laboratory detection kit
- Bacteria rapid diagnostic test
- Cell assay kit
- Genetic test kit
- Nasopharyngeal assay kit
- Clinical rapid diagnostic test
- Nasal detection kit
- Nucleic acid assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.