- Laboratory
- Laboratory medicine
- Medium reagent
- ITEL Telecomunicazioni
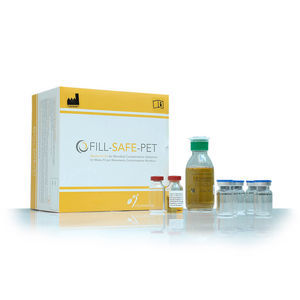
Reagent medium reagent kit FILL-SAFEfor microbial contamination
Add to favorites
Compare this product
Characteristics
- Type
- reagent medium
- Applications
- for microbial contamination
Description
FILL-SAFE allows three simulations of a conventional radiopharmaceutical preparation. The kit enables aseptic process validation in terms of operator skills and of the manual procedures adopted according to Chapter 11 of “Good Preparation Procedures in Nuclear Medicine”.
Media-Fill procedure is closely linked to the operating procedures of each individual department and cannot be generalized. The leaflet suggests a possible operational sequence, that must be approved by the responsible personnel of the Nuclear Medicine Department.
At the end of the three simulations, all of the samples collected may be shipped to ITELPHARMA for incubation at the temperature of 22.5°C/32.5°C for a total of 14 days. The sterile samples then undergo a fertility test performed to verify the compliance of the culture medium.
Upon completion of the tests, the quality control department issues an analysis certificate containing the results of the analytical tests performed using the kit.
Il kit contiene:
4 100 ml vials of TSB culture medium
15 sterile, non-pyrogenic, vacuum packed vials
Identification labels,
Instruction leaflet
CD-ROM containing a standard format for Protocol and Validation Reports
Analysis certificate guaranteeing product quality
Catalogs
No catalogs are available for this product.
See all of ITEL Telecomunicazioni‘s catalogs*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.