- Secondary care
- Cardiology
- Coronary stent
- Japan Stent Technology

- Products
- Catalogs
- News & Trends
- Exhibitions
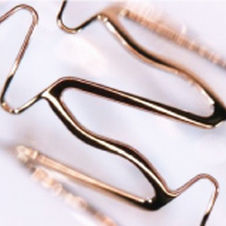
Coronary stent MOMO®cobalt-chromium
Add to favorites
Compare this product
Characteristics
- Type
- coronary arteries
- Material
- cobalt-chromium
Description
The product went on sale in Europe in February 2010 under the name “MOMO® Coronary Stent System” after obtaining CE Marking certification in September 2009.
Intended for use by patients with ischemic heart disease symptoms such as myocardial infarction, this medical device is designed to be inserted into the heart’s blood vessels (coronary arteries) at the site of disease-causing constriction, where it restores the original level of blood flow by physically widening the vessel.
MOMO® is a bare metal stent—that is, a type of coronary artery stent that does not use drugs. Its surface is coated with a nano-level coating of diamond-like carbon, a highly biocompatible substance that is also used in mechanical artificial heart valves, that has been optimized for use in coronary artery stents. MOMO® minimizes irritation at the implant site thanks to a closed cell design that offers an exceptional balance of strength and flexibility and world-class thinness by its cobalt-chromium construction.
Clinical trials of MOMO® Coronary Stent System have yielded excellent results and we would like to work to improve the Quality of Life for people with our product.
Related Searches
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.

