- Laboratory
- Laboratory medicine
- Lung cancer test kit
- Jiangsu Macro micro-test Medical Technology
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
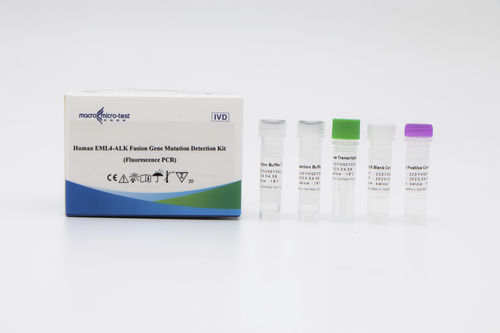
Genetic mutation test kit HWTS-TM006Afor lung cancerEML4-ALK fusion geneFFPE tissues

Add to favorites
Compare this product
Characteristics
- Applications
- for genetic mutations, for lung cancer
- Tested parameter
- EML4-ALK fusion gene
- Sample type
- FFPE tissues
- Analysis mode
- for RT-PCR, fluorescence
Description
This kit is used to qualitatively detect 12 mutation types of EML4-ALK fusion gene in samples of human nonsmall cell lung cancer patients in vitro (Table 1). The test results are for clinical reference only and should not be used as the sole basis for individualized treatment of patients. Clinicians should make comprehensive judgments on the test results based on factors such as the patient's condition, drug indications, treatment response, and other laboratory test indicators.
Lung cancer is the most common malignant tumor worldwide, and 80%~85% of the cases are nonsmall cell lung cancer (NSCLC). Gene fusion of echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) is a novel target in NSCLC, EML4 and ALK are respectively located in human the P21 and P23 bands on chromosome 2 and are separated by approximately 12.7 million base pairs [1]. At least 20 fusion variants have been found, among which the 12 fusion mutants in Table 1 are common, where mutant 1 (E13; A20) is the most common one, followed by mutants 3a and 3b (E6; A20), accounting for about 33% and 29% of patients with EML4-ALK fusion gene NSCLC, respectively [2]. ALK inhibitors represented by Crizotinib are small-molecule targeted drugs developed for ALK gene fusion mutations. By inhibiting the activity of the ALK tyrosine kinase region, blocking its downstream abnormal signaling pathways, thereby inhibiting the growth of tumor cells , to achieve targeted therapy for tumors [3].
Catalogs
No catalogs are available for this product.
See all of Jiangsu Macro micro-test Medical Technology‘s catalogsOther Jiangsu Macro micro-test Medical Technology products
FLUORESCENCE PCR
Related Searches
- Reagent kit
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Liquid reagent kit
- Plasma assay kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Infectious disease assay kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Respiratory infection test kit
- Rapid virus test
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Optical assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.




























































































































