- Laboratory
- Laboratory medicine
- COVID-19 test kit
- Jiangsu Macro micro-test Medical Technology

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
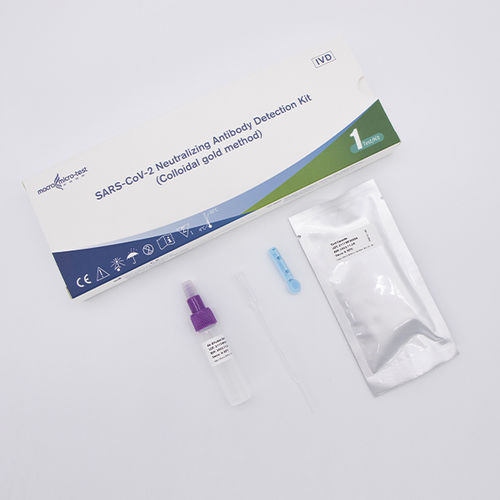
Infectious disease test kit HWTS-RT077BCOVID-19for neutralizing antibodySARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- for infectious diseases, COVID-19
- Tested parameter
- for neutralizing antibody
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- whole blood
- Analysis mode
- immunochromatographic
- Format
- cassette, strip
- Other characteristics
- custom, self-test
Description
This product is applicable to the qualitative detection of novel coronavirus (SARS-CoV-2) neutralizing antibodies in patients who have recovered from naturally infected with SARS- CoV-2 or those who have been vaccinated.
■ Intended Use
This product is applicable to the qualitative détection of novel coronavirus (SARS-CoV-2) neutralizing antibodies in patients who have recovered from naturally infected with SARS-CoV-2 or those who have been vaccinated.
Human immunity is assessed by testing for the presence of novel Coronavirus (SARS-CoV-2) neutralizing antibodies in sérum, plasma and whole blood samples of people infected with COVID-19 or immunized with vaccine.
■ Necessary
Neutralizing antibody détection has always played an important rôle in evaluating the level of human immune response after vaccination in the évaluation of vaccine effectiveness.
Neutralizing antibodies can prevent the Novel Coronavirus from binding to receptors on the cell surface, thereby directly preventing the virus from infecting cells. This means that the body could fight off new infections in the future with neutralizing antibodies.
It can accurately detect the presence of neutralizing antibodies and their ability to determine whether a body has built up immunity against the Novel coronavirus.
Catalogs
No catalogs are available for this product.
See all of Jiangsu Macro micro-test Medical Technology‘s catalogsOther Jiangsu Macro micro-test Medical Technology products
Colloidal Gold Chromatography
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Optical assay kit
- Clinical assay kit
- Infectious disease rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.