- Laboratory
- Laboratory medicine
- Enterovirus test kit
- Jinan Babio Biotech
Autoimmune disease test kit IgMenterovirusserum
Add to favorites
Compare this product
Characteristics
- Applications
- for autoimmune diseases
- Tested parameter
- IgM
- Micro-organism
- enterovirus
- Sample type
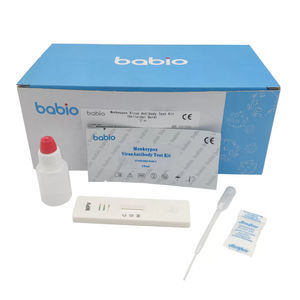
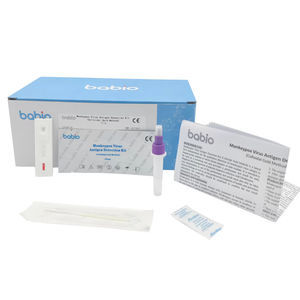
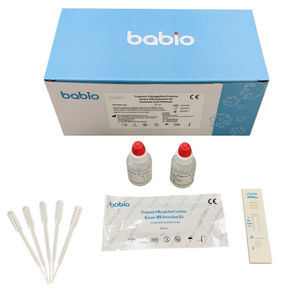
- serum, plasma, whole blood, surface
- Result display time
15 min
- Sample volume
0.01 ml
(0.00034 US fl oz)
Description
Enterovirus 71 (EV71)-IgM Detection Kit (Colloidal Gold Method) is a lateral flow immunoassay for the qualitative detection of IgM-class antibodies to human Enterovirus 71 (EV71) in serum, plasma or whole blood samples. It is intended to be used as a screening test and provides a preliminary test result for early diagnosis and management of patients related to infection with EV71.
【TEST PRINCIPLE】
This kit adopts colloidal gold-immunochromatography assay (GICA).
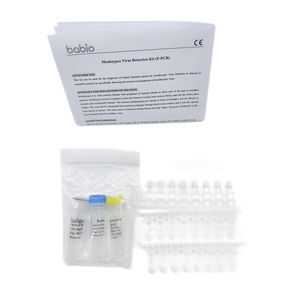
The test card contains:
1. Colloidal gold-labeled antigen and quality control antibody complex.
2. Nitrocellulose membranes immobilized with one test line (T line) and one quality control line (C line).
When an appropriate amount of sample is added to the sample well of the test card, the sample will move forward along the test card under capillary action.
If the sample contains an IgM antibody of EV71, the antibody will bind to the colloidal gold-labeled EV71 antigen, and the immune complex will be captured by the monoclonal anti-human IgM antibody immobilized on the nitrocellulose membrane to form a purple/red T line , showing that the sample is positive for IgM antibody.
【SHELF LIFE AND STORAGE】
1. The original packaging should be stored in a dry place at 2-30°C and protected from light.
2. The shelf life of the test kit is 2 years from date of manufacture. Refer to the product labels for stated expiration date.
3. The original packaging can be transported at 2-37℃ for 20 days.
4. After opening the inner package, the test card will become invalid due to moisture absorption, please use it within 1 hour.
Catalogs
No catalogs are available for this product.
See all of Jinan Babio Biotech‘s catalogsRelated Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Clinical assay kit
- Reagent medium reagent kit
- Whole blood rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.