- Secondary care
- Orthopedic surgery
- Sternal closure bone cerclage wiring
- Kontour Medical Technology
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
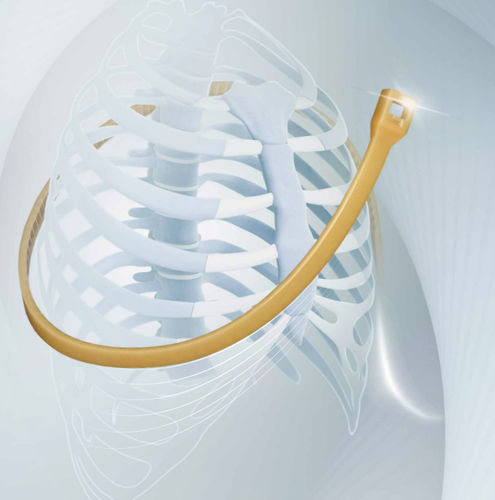
Sternal closure bone cerclage wiring SternoEaZip®monofilament polymer

Add to favorites
Compare this product
Characteristics
- Application
- for sternal closure
- Type of wiring
- monofilament polymer
Description
PEEK-OPTIMA® polymer, provided by Invibio, is now recognized as a high performance biomaterial in worldwide. It is formulated to meet the most exacting in-vivo criteria and present excellent mechanical performance, plasticity, chemical resistance and biocompatibility. It is also capable to be repeatedly sterilized without impairing performance and behaves highly radiolucent CT/MRI imaging without scattering or artifacts. PEEK-OPTIMA polymer has been widely utilized in cardiovascular, dental, neurological and orthopaedic implants and globally accumulated more than 9 million implant cases
Excellent Biocompatibility
Good biological characteristics and histocompatibility Capable to sustain repeated steam, ETO sterilization and Gamma irradiation Non-cytotoxic, non-carcinogenic, non-conductive,hydrolysis resistance and corrosion resistance
Tried and Tested
Test material implanted in paravertebral muscle for one year caused virtually no response-mild fibrosis, or in some cases a light fibrous capsule. There was no muscle degeneration, nor necrosis, or any other significant change.
PEEK-OPTIMA® Biocompatibility Tests
• Genotoxicity ISO 10993-3
• Hemolysis (Extract) ISO 10993-4
• Cytotoxicity ISO 10993-5
• Biostability Local Effects of Implantation ISO 10993-6
• Sensitization ISO 10993-10
• Pyrogenicity ISO 10993-11
• Chemical Analysis ISO 10993-18
• USP Plastic Class VI Systemic Toxicity Study
• USP Plastic Class VI Intracutaneous Toxicity Study
PEEK-OPTIMA® is an inherently pure inert material. Extensive biocompatibility testing demonstrated no evidence of cytotoxicity, systemic toxicity, irritation or any macroscopic reaction response.
VIDEO
Catalogs
No catalogs are available for this product.
See all of Kontour Medical Technology‘s catalogsExhibitions
Meet this supplier at the following exhibition(s):


Other Kontour Medical Technology products
Cardiovascular Surgery
Related Searches
- Bone plate
- Compression plate
- Metallic compression plate
- Locking compression plate
- Titanium compression plate
- Interbody fusion cage
- PEEK interbody fusion cage
- Lumbar interbody fusion cage
- Anterior interbody fusion cage
- Screw-rod unit
- Posterior spinal fusion system
- Cervical interbody fusion cage
- Adult spinal fusion system
- Posterior interbody fusion cage
- Transforaminal interbody fusion cage
- Thoraco-lumbar spinal fusion system
- Cranial fixation system
- Non-absorbable cranial fixation system
- Cranial implant
- Bone cerclage
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.



