Medical software PAS-X Content Suite for the pharmaceutical industrydata managementdesign
Add to favorites
Compare this product
Characteristics
- Applications
- medical, for the pharmaceutical industry
- Function
- data management, for equipment control, reporting, design, data processing
Description
With the Werum PAS-X Content Suite, you can quickly set up PAS-X MES systems for different industries such as pharma, biotech, and cell & gene therapy. For this purpose, our Content Suite provides you with pre-parameterized content supporting you whilst you configure your specific Werum PAS-X MES system. You can rapidly implement your business processes with the help of our consultants and best-practice content for a wide range of applications. This saves you time and reduces risks.
The Werum PAS-X Content Suite provides you with a total of 16 content packages for four application areas:
Administration Templates
Process Libraries
Integration Libraries
Equipment Libraries
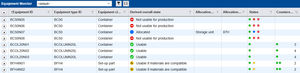
Equipment State Diagrams — for the design and documentation of equipment status diagrams. The package also includes ready-to-use best practice status diagrams for e.g. cleaning, assembly, and sterilization
Master Data: the right way — describes in detail the master data required for using the PAS-X MES system. It explains specifics regarding the master data and considers the sequence for creating master data
Reports & Labels and Enhanced Label — contains GMP-compliant templates for reports and labels as well as pre-configured reports, such as MBR reports and batch reports
Rights & Roles — supports the configuration of the PAS-X MES user rights based on global, GMP-compliant profiles and specific roles such as operator, supervisor, and QA/QM personnel
Workflows — delivers information about the best practices to adapt the release workflows, e.g. MBRs and BRRs (only relevant for PAS-X MES 3.1.8)
Simple launch
Quick, easy, and secure implementation of your Werum PAS-X MES
Catalogs
No catalogs are available for this product.
See all of Körber Pharma‘s catalogsRelated Searches
- Analysis software
- Control software
- Reporting software
- Scheduling software
- Design software
- Online software
- Data management software
- Körber Pharma manufacturing software
- Real-time software
- Data processing software
- Körber Pharma software for the pharmaceutical industry
- Equipment control software
- Production environment software
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.