- Secondary care
- Gyneco-obstetrics
- Copper-T intrauterine device
- Laboratoire CCD
Copper-T intrauterine device Etherena® T Cu 380A
Add to favorites
Compare this product
fo_shop_gate_exact_title
Characteristics
- Type
- copper-T
Description
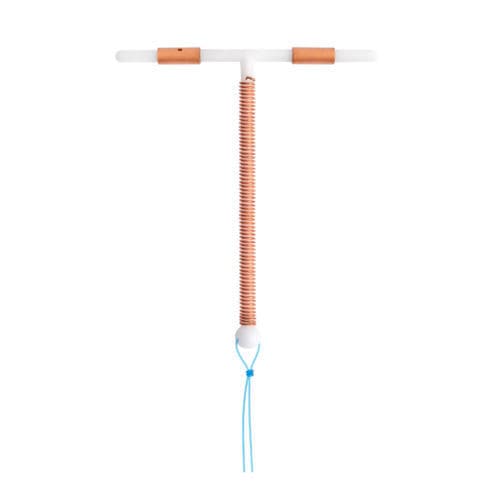
380 mm² copper intrauterine contraceptive device (IUD) with insertion system and disposable hysterometer.
The T-IUD is non-hormonal, it prevents implantation in the uterus of the fertilized oocyte. This activity is reinforced in an ancillary manner by the presence of copper. Copper has a cytotoxic effect on gametes which causes damage to sperm, and inhibits fertilization. The Etherena® T-IUD is effective from the day it has been inserted.
Description
A reversible long-term contraceptive method (up to 10 years) which is indicated in uniparous and multiparous women for:
intra-contraception usual first-line uterine,
emergency postcoital intrauterine contraception (within 5 days of unprotected or poorly protected intercourse)
postcoital intrauterine contraception partum and post-abortion (within 48 hours post-partum or post-abortion or after a complete involution of the uterus).
Composition
The case includes: 1 T-IUD with insertion device, 1 patient card, 1 doctor’s leaflet with a detachable coupon for the patient, 1 disposable hysterometer. Etherena T Cu 380 A consists of a T-shaped body made of polyethylene and barium sulfate. A copper wire is wound around the vertical axis. The 2 horizontal arms have copper sleeves. The total copper surface, which is 380 ± 38 mm2. A monofilament suture in nylon is attached to the T-structure ball, at the base of the vertical axis.
Etherena T Cu 380 A measures 32mm wide × 36 mm long loaded in the insertion system and disposable hysterometer, unit box. ACL code: 3700111400184 Class III medical device.
Related Searches
- Catheter
- Cannula
- 18G needle
- Sterile needle
- 16G needle
- Puncture needle
- Suction cannula
- Peripheral catheter
- Diagnostic catheter
- Double-lumen catheter
- Echogenic needle
- Disposable cannula
- Dilatation sheath
- Silicone catheter
- Injection cannula
- Female catheter
- Intrauterine catheter
- IUD
- Copper intrauterine device
- Cervical dilator
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.







