- Laboratory
- Sample management
- Steam sterilization bioindicator
- Liofilchem S.r.l.
Steam sterilization biological indicator 91101for the pharmaceutical industry

Add to favorites
Compare this product
Characteristics
- Type
- for steam sterilization
- Applications
- for the pharmaceutical industry
Description
USP (United States Pharmacopoeia) and EP (European Pharmacopoeia) standards, recommend the use of bioindicators during steam sterilization processes of pharmaceuticals, drugs in vials, culture media, medical instruments and more.
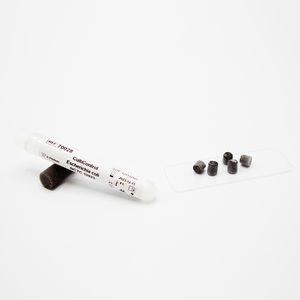
STERILtest GST E6/E5 self-contained are made of a thermoplastic tube, with a cup permeable to steam, that contains an ampoule with a nutrient broth with a pH indicator and a disc impregnated with Geobacillus stearothermophilus (ATCC 7953) spores in predefined concentrations (E6 = 1-5 x 106 UFC/disc; E5 =1-5 x 105 UFC/disc). These bioindicators are used for regular control of steam sterilization cycles and control of any steam autoclave functionality.
Biological indicators STERILtest GST E6/E5 self-contained are produced under strictly controlled conditions in order to satisfy the requirements indicated in the USP current edition and in accordance with ISO 11138 and EN 866 standards.
VIDEO
Catalogs
Biological Indicators
4 Pages
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.