Cardiac Holter monitor software EUROHOLTERanalysismanagementreporting
Add to favorites
Compare this product
Characteristics
- Applications
- for cardiac Holter monitors
- Function
- analysis, management, reporting, for control, recording, traceability
Description
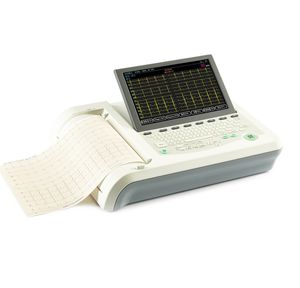
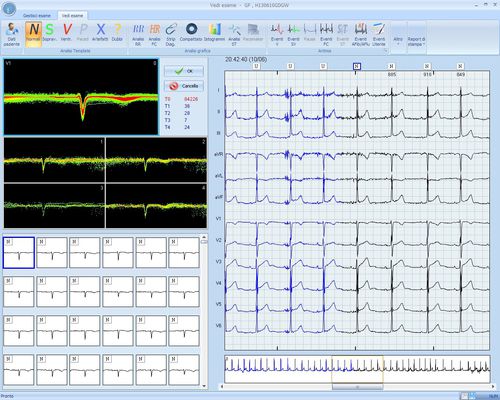
The LUMED® EUROHOLTER software has all the most advanced functions for analysis and validation of recordings: from the classic Compacted to QT/QTc, Atrial Fibrillation etc. analysis, up to Sleep Apnea analysis. The commands are accessible with both the mouse and the keyboard and reporting is therefore extremely fast.
Catalogs
No catalogs are available for this product.
See all of LUMED‘s catalogsExhibitions
Meet this supplier at the following exhibition(s):

*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.