- Laboratory
- Sample management
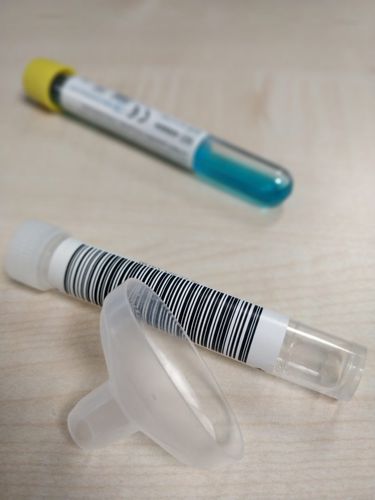
- Saliva collection kit
- magtivio B.V.
Saliva collection kit SafeQnasopharyngeal
Add to favorites
Compare this product
Characteristics
- Sample type
- saliva, nasopharyngeal
Description
Fast and timely detection of coronavirus infection could prevent the closure of your company or institution. Detection of coronavirus is currently mainly done with nasopharyngeal swabs. These can be unpleasant or painful. In addition, medical personnel is required for taking the swab sample. A saliva test addresses these shortcomings by being painless and simple to perform. Moreover, many studies show the suitability of saliva for the detection of SARS-CoV-2 when performed on a regular basis (once or twice a week).
Repetitive saliva testing can help identify people who spread the virus but don’t show any symptoms. The technique is therefore complementary to existing testing strategies and can be used as a third wall of defence to keep companies and organizations open. SafeQ Saliva Collection Kit with its special inactivation and preservation buffer also overcomes a common challenge: SARS-CoV-2 infection of lab operators when handling patients samples.
Features
Pain-free sample collection
CE-IVD marked device
Easy self-sampling, no need for medical staff
Inactivation buffer allows for complete inactivation of SARS-CoV-2:
it abrogates the infectious potential of the collected saliva
Safe and stable preservation of RNA up to 30 days when samples are stored at 2-25°C and up to 8 days when stored at 37°C. No cold chain required
Buffer has a distinct color as visual pipetting control
Procedure
The saliva collection procedure starts by spitting in the funnel, placed on the collection device, until the amount of liquid saliva reaches the indicated line.
Catalogs
SafeQ Saliva Collection Kit
2 Pages
Product Catalog 2023
52 Pages
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.



