- Furniture, Logistics
- Logistics, Service, Storage
- Transfer cabinet
- MDG Engineering S.r.l.
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
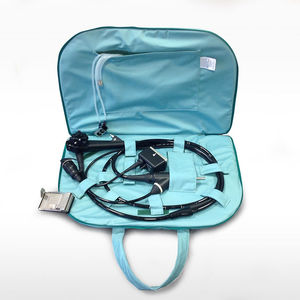
Transfer cabinet AES-CERBERUS®for endoscopesfor flexible endoscopesprobe
Add to favorites
Compare this product
Characteristics
- Type
- transfer
- Use
- for endoscopes, for flexible endoscopes, probe
- Facility
- intensive care
- Components
- on casters, with tray
- Features
- automated
- Material
- stainless steel
- Color
- gray
- Options and accessories
- RFID technology, for dry storage
- Number of doors
- 1-door
- Number of units
- 1-unit
Description
AES-CERBERUS® is a class I Medical device which complies with EN 16442:2015 and Medical Device Regulation 2017/745. Its system is patented and validated with cold plasma technology for the elimination of pathogens. An innovative solution which guarantees the maximum protection of enveloped material that needs to be transported and stored under aseptic conditions.
AES-CERBERUS® was created both to centralize the conservation process and to facilitate daily usage in high and low workflow wards, such as the departments of endoscopy, ENT, bronchoscopy, urology, intensive care and cardiology (for TEE probes).
The machine employs vacuum technology alternated with a patented system involving cold plasma injections with generation of free radicals. Thanks to its certified microbicidal action (virus, bacteria and other pathogens) it can keep the high-level disinfection status of enveloped instruments for up to 800 hours. This eliminates the risk of air-borne contamination and the risk of cross-contamination during enveloping procedures.
Designed to be compatible with every brand and model of endoscope, transesophageal probe (TEE) and other medical devices subject to high-level disinfection, without the need for connections to the endoscope channels.
Traceability is ensured by automatic printing of cycles on thermal paper (adhesive and triplex thermal paper roll optional) or by data transfer via Ethernet (optional software). Equipped with specific cycles for the different types of instruments that need to be stored. Every cycle is validated through automatic measurement of the plasma concentration inside the bag.
Catalogs
AES-CERBERUS
2 Pages
Related Searches
- Medical cart
- Logistics cart
- MDG hospital cabinet
- Medical device cart
- MDG stainless steel cabinet
- Linen trolley
- MDG 2-door cabinet
- Medical linen trolley
- MDG 1-door cabinet
- MDG cabinet with drawer
- Single-module cabinet
- Transport linen trolley
- MDG gray cabinet
- MDG instrument cabinet
- MDG laboratory cabinet
- Cart with door
- MDG medical device cabinet
- Mobile cabinet
- Dirty linen trolley
- Rack
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.