- Medical Technical Facilities
- Monitoring
- Monitoring ECG electrode
- Med-link Electronics Tech
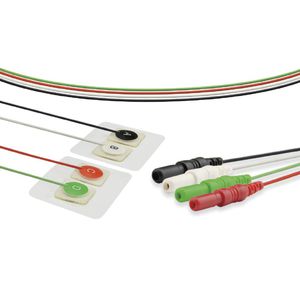
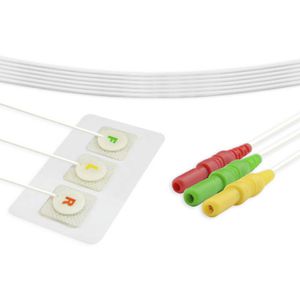
Monitoring ECG electrode V0014ILchestdisposable
Add to favorites
Compare this product
Characteristics
- Applications
- monitoring
- Zone of use
- chest
- Use
- disposable
Description
Regulatory compliance: FDA, CE, ISO 80601-2-61:2011, ISO10993-1, 5, 10:2003E, TUV, RoHS Compliant
Patient Size: Infant/Neonate
Hypoallergenic: YES
Radiolucent : NO
Shape: Round
Applicable : ECG diagnosis,ECG monitoring,ECG workstation;Cardiopathy/NICU
Size: Φ25mm,Φ30mm
Gel type: Hydrogel
Electrode Location: Center
Electrode Material : Stainless steel
Backing Material: Non-woven
Latex-free : Yes
Times of use: Only use for single patient
Packaging Type: Box
Packaging Unit: 250pcs
Shelf life: 2 years
Weight : /
Sterile: Sterilization available
Catalogs
No catalogs are available for this product.
See all of Med-link Electronics Tech‘s catalogsExhibitions
Meet this supplier at the following exhibition(s):
Related Searches
- SpO2 monitor
- SpO2 sensor
- Reusable electrode
- Hospital pulse oximeter
- Temperature probe
- Fingertip pulse oximeter
- Monitoring probe
- Disposable electrode
- Blood pressure cuff
- Fingertip SpO2 sensor
- Reusable SpO2 sensor
- ECG electrode
- IBP cable
- Wireless pulse oximeter
- Monitoring ECG electrode
- 1-tube cuff
- Hand-held SpO2 monitor
- Disposable SpO2 sensor
- Medical electrode
- OLED pulse oximeter
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.