- Medical Technical Facilities
- Monitoring
- Monitoring ECG electrode
- Med-link Electronics Tech
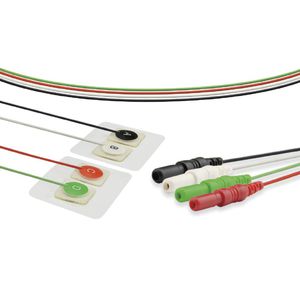
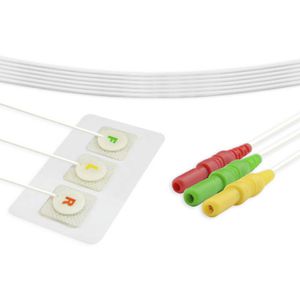
Monitoring ECG electrode COE0002Adisposableradiolucent
Add to favorites
Compare this product
Characteristics
- Applications
- monitoring
- Use
- disposable
- Other characteristics
- radiolucent
Description
egulatory compliance - FDA, CE, ISO 80601-2-61:2011, ISO10993-1, 5, 10:2003E, TUV, RoHS Compliant
Patient Size - Adult
Hypoallergenic - NO
Radiolucent - NO
Shape - Polygon
Applicable - Anesthesiology, ICU, Operating room
Size - 110*160mm
Gel type - Wet Del
Electrode Material - Stainless steel
Backing Material - Foam
Latex-free - Yes
Times of use - Only use for single patient
Packaging Type - Box
Packaging Unit - 25pcs
Shelf life - 2 years
Weight - /
Sterile - Sterilization available
Catalogs
No catalogs are available for this product.
See all of Med-link Electronics Tech‘s catalogsExhibitions
Meet this supplier at the following exhibition(s):

Related Searches
- SpO2 monitor
- SpO2 sensor
- Reusable electrode
- Hospital pulse oximeter
- Temperature probe
- Fingertip pulse oximeter
- Monitoring probe
- Disposable electrode
- Fingertip SpO2 sensor
- Blood pressure cuff
- Reusable SpO2 sensor
- ECG electrode
- IBP cable
- Wireless pulse oximeter
- Monitoring ECG electrode
- 1-tube cuff
- Hand-held SpO2 monitor
- Disposable SpO2 sensor
- OLED pulse oximeter
- Medical electrode
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.