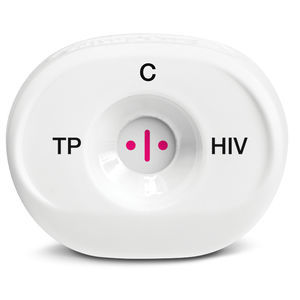
Rapid AIDS test revealHIVfor antibodiesHIVserum

Add to favorites
Compare this product
Characteristics
- Applications
- AIDS
- Tested parameter
- for antibodies
- Micro-organism
- HIV
- Sample type
- serum, plasma, whole blood, clinical
- Specificity
99.7 %
- Sensitivity
99.8 %
Description
In 3 easy steps Reveal HIV instantly detects antibodies to HIV-1 and HIV-2 in whole blood, serum or plasma.
This test, available in three formats, is suited for a broad range of testing environments from the point-of-care to the laboratory. Testing applications for Reveal HIV include public health programs, Voluntary Testing & Counselling Centers (VCTs), pharmacies, physician offices, immigration screening, occupational exposures, convenience care clinics, correctional facilities, and more.
The FDA-approved Reveal® G4 is the next generation of rapid testing for HIV using MedMira’s patented Rapid Vertical Flow (RVF) Technology™.
Now able to test fingerstick and venipuncture whole blood, Reveal G4 helps healthcare providers test patients not only in laboratories and hospitals but also in point-of-care settings.
Reveal G4 is approved for clinical laboratory use only.
Product Features:
Works with serum, plasma, and venipuncture or fingerstick whole blood specimens.
Simple procedure with instant, easy-to-interpret results.
Built-in procedural and reagent control line.
Room-temperature storage.
No timers or specialized equipment needed.
External test controls available as an accessory*
REVEAL HIV – CANADA
In Canada, Reveal Rapid HIV Antibody Test is approved by Health Canada for Laboratory Use Only. Customers in the Canadian healthcare industry, including hospitals, reference laboratories, and sexual health clinics with onsite laboratories use the test with serum and plasma specimens.
Qty. 30 tests per box
This format is ideally suited for laboratories using serum and plasma specimens, and batch testing.
VIDEO
Catalogs
No catalogs are available for this product.
See all of MedMira‘s catalogsRelated Searches
- Assay kit
- Blood rapid diagnostic test
- Immunoassay rapid diagnostic test
- Virus rapid diagnostic test
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Optical assay kit
- Whole blood rapid diagnostic test
- Rapid respiratory infection test
- Research assay kit
- Antigen assay kit
- COVID-19 rapid diagnostic test
- Bacteria rapid diagnostic test
- Clinical rapid diagnostic test
- Nasal rapid diagnostic test
- Laboratory rapid diagnostic test
- Influenza A rapid diagnostic test
- Influenza B rapid diagnostic test
- Rapid throat test
- Colorimetric assay kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.