- Medical Technical Facilities
- Intensive care
- Double blood bag
- MITRA Industries
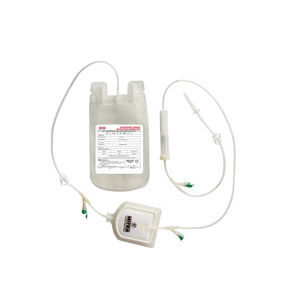
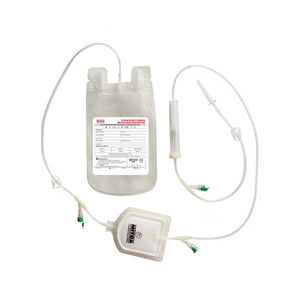
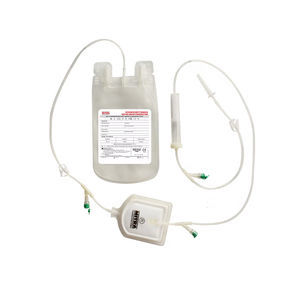
Double blood bag CPDCPDA-1PVC
Add to favorites
Compare this product
Characteristics
- Type
- double
- Anticoagulant
- CPDA-1, CPD
- Material
- PVC
- Function
- for red blood cells, for blood plasma
- Norms
- ISO 3826
- Volume
350 ml, 400 ml, 450 ml, 500 ml
(11.83 US fl oz, 13.53 US fl oz, 15.22 US fl oz, 16.91 US fl oz)
Description
Mitra Blood Bags are designed for the collection, processing storage and administration of whole
human blood and it’s components.
2. Blood taking needle
3. Needle Hub
4. PVC Tube
5. External Ports
6. Means of closure
7. Side Slits
8. Bottom Slits
9. Labels
TECHNICAL SPECIFICATIONS
• Inner bag maintains the sterility.
• No risk of contamination & air embolism with all leak proof.
• Slits on the both side to accommodate 5-10 ml volume test tube & at the bottom of bag to hang the blood bag during transfusion.
• Flexible, non-sticking, transparent, leak proof tubing having multiple printed & ID/Segment number.
• 16 G ultra thin walled, needle triple bevel design, sharp, rust proof, tightly fixed with hub & tamper proof needle cover.
• Tamper proof & easily accessible external port.
• Hot melt technology non-peel able, heat sealed label with transparent adhesive remarked attached between room temperature 0 to -80 C.
• 3 years shelf life from the date of manufacturing.
• Maintain biochemical parameters of PRBC up to 35 days of storage in plain & plasma (FFP) up to 1 year.
• Protective dual packaging (CPP & Aluminum)
• Individual plastic blood bags packed in plastic CPP pouch & such 6 in plain plastic pouches packed in an aluminum foil pack. 10 (Plain) such aluminum foil packs further packed in corrugated boxes.
Catalogs
No catalogs are available for this product.
See all of MITRA Industries‘s catalogsRelated Searches
- Laboratory filter
- Sterile filter
- Blood filter
- Blood bag
- PVC blood bag
- Leukoreduction filter
- CPDA-1 blood bag
- CPD blood bag
- Single blood bag
- Quadruple blood bag
- Blood bank filter
- Red blood cells filter
- Triple blood bag
- Double blood bag
- Whole blood filter
- Blood plasma blood bag
- Platelet blood bag
- Red blood cell blood bag
- ISO 3826 blood bag
- Blood bag filter
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.