- Laboratory
- Laboratory medicine
- Serum reagent
- MP Biomedicals
- Products
- Catalogs
- News & Trends
- Exhibitions
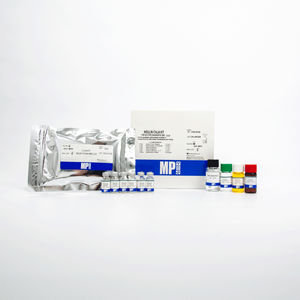
Insulin reagent kit 07M60102serumdiagnosticfor ELISA
Add to favorites
Compare this product
Characteristics
- Type
- serum
- Applications
- diagnostic, for ELISA
- Tested parameter
- for insulin
Description
The Insulin ELISA Kit test is intended to be used for the quantitative determination of insulin levels in human serum.
Usage Statement
FDA cleared for In Vitro Diagnostic (IVD) use in the United States. CE Marked for IVD use outside the United States.
Key Applications
Endocrinology
Analyte / Target - Insulin
Base Catalog Number - M60102
Diagnostic Platforms - EIA/ELISA
Diagnostic Solutions - Endocrinology
Disease Screened - Insulin
Evaluation - Quantitative
Pack Size - 96 Wells
Sample Type - Serum
Sample Volume - 50 ul
Species Reactivity - Human
Usage Statement - FDA cleared for In Vitro Diagnostic (IVD) use in the United States. CE Marked for IVD use outside the United States.
Catalogs
No catalogs are available for this product.
See all of MP Biomedicals‘s catalogsRelated Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Research reagent kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Protein reagent kit
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Enzyme reagent kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Whole blood detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.