- Laboratory
- Laboratory medicine
- Serum reagent
- MP Biomedicals
- Products
- Catalogs
- News & Trends
- Exhibitions
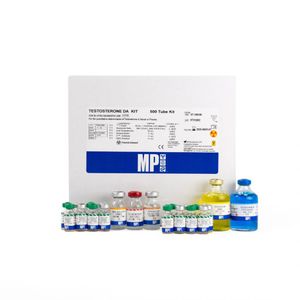
Testosterone reagent kit 07M3775Aserumplasmadiagnostic
Add to favorites
Compare this product
Characteristics
- Type
- serum, plasma
- Applications
- diagnostic
- Tested parameter
- testosterone
- Method
- chemiluminescence
Description
The Testosterone Chemiluminescence Immunoassay kit is intended for the measurement of Total Testosterone in human serum or plasma.
Usage Statement
FDA cleared for In Vitro Diagnostic (IVD) use in the United States. CE Marked for IVD use outside the United States.
Analyte / Target - Testosterone
Base Catalog Number - M3775A
Diagnostic Platforms - ChLIA
Diagnostic Solutions - Endocrinology
Disease Screened - Testosterone
Evaluation - Quantitative
Pack Size - 1 96 Wells
Sample Type - Plasma, Serum
Sample Volume - 10 µL
Sensitivity - 0.026 ng/mL
Species Reactivity - Human
Usage Statement - FDA cleared for In Vitro Diagnostic (IVD) use in the United States. CE Marked for IVD use outside the United States.
Catalogs
No catalogs are available for this product.
See all of MP Biomedicals‘s catalogsRelated Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Molecular biology reagent kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Research reagent kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Protein reagent kit
- Diagnostic reagent kit
- Laboratory reagent kit
- Rapid lateral flow test
- Enzyme reagent kit
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Whole blood detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.