- Laboratory
- Laboratory medicine
- COVID-19 test kit
- Nanjing Liming Bio-products Co., Ltd.

- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
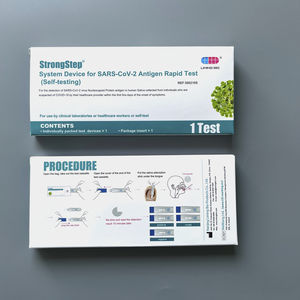
COVID-19 test kit StrongStep®SARS-COV-2influenza Ainfluenza B
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Micro-organism
- SARS-COV-2, influenza A, influenza B
- Sample type
- nasopharyngeal, laboratory, nasal
- Analysis mode
- for real-time PCR, RT-qPCR
- Result display time
2 h
Description
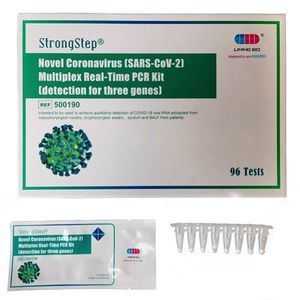
StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for simultaneous qualitative detection and differentiation of SARS-CoV-2,Influenza A virus and Influenza B virus RNA in healthcare provider-collected nasal and nasopharyngeal swab or oropharyngeal swab specimens and self-collected nasal or oropharyngeal swab specimens(collected in a healthcare setting with instruction by a healthcare provider) from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. RNA from SARS-CoV-2, influenza A, and influenza B is generally detectable in respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, influenza A, and/or influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude infection from SARS-CoV-2, influenza A, and/or influenza B and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. StrongStep® SARS-CoV-2 & Influenza A/B Multiplex Real-Time PCR Kit is intended for use by qualified clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR assays and in vitro diagnostic procedures.
contents
Kit Components
• SARS-CoV-2 and Influenza A/B RT-qPCR reagents
• Positive control
• PCRCAP
Catalogs
COVID-19 leaflets
32 Pages
Other Nanjing Liming Bio-products Co., Ltd. products
SARS-CoV-2
Related Searches
- Assay kit
- Solution reagent kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Histology reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- COVID-19 detection kit
- Rapid respiratory infection test
- Real-time PCR test kit
- Urine rapid diagnostic test
- Clinical reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.