- Laboratory
- Laboratory medicine
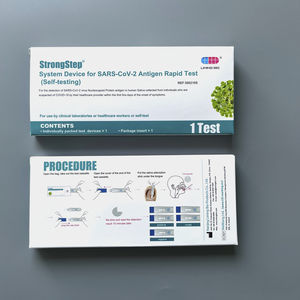
- COVID-19 rapid test
- Nanjing Liming Bio-products Co., Ltd.
COVID-19 rapid test 502090IgGIgMSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- IgG, IgM
- Micro-organism
- SARS-COV-2, coronavirus
- Sample type
- serum, plasma, whole blood
- Analysis mode
- CLIA
Description
The test is limited in the US to distribution to laboratories certified by CLIA to perform high complexity testing.
This test has not been reviewed by the FDA.
Negative results do not preclude acute SARS-CoV-2 infection.
Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-2 infection.
Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
They can also identify whether they have previously been infected with the SARS-CoV-2 virus and have recovered.This test is only authorized to detect SARS-CoV-2 specific IgM and IgG antibodies.IgG and lgM antibodies to 2019 Novel Coronavirus can be detected with 2-3 weeks after exposure. Negative results do not preclude acute SARS-CoV-2 infection. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. lgG remains positive, but the antibody level drops overtime. It is not applicable to any other viruses or pathogens, and the results should not be used to diagnose or rule out SARS-CoV infection or inform the status of infection.
If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
INTENDED USE
TheStrongStep® SARS-CoV-2 IgM/IgG Test is a rapid immuno-chromatographic assay for the simultaneous detection of IgM and IgG antibodies to SARS-CoV-2 virus in human whole blood, serum or plasma. The assay is used as an aid in the diagnosis of COVID-19.
Catalogs
COVID-19 leaflets
32 Pages
Exhibitions
Meet this supplier at the following exhibition(s):

Other Nanjing Liming Bio-products Co., Ltd. products
SARS-CoV-2
Related Searches
- Assay kit
- Solution reagent kit
- Blood rapid diagnostic test
- Diagnostic reagent kit
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Molecular test kit
- Cassette rapid diagnostic test
- Rapid virus test
- Respiratory infection test kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Histology reagent kit
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- COVID-19 assay kit
- Rapid respiratory infection test
- Real-time PCR test kit
- Urine rapid diagnostic test
- Clinical reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.