- Laboratory
- Laboratory medicine
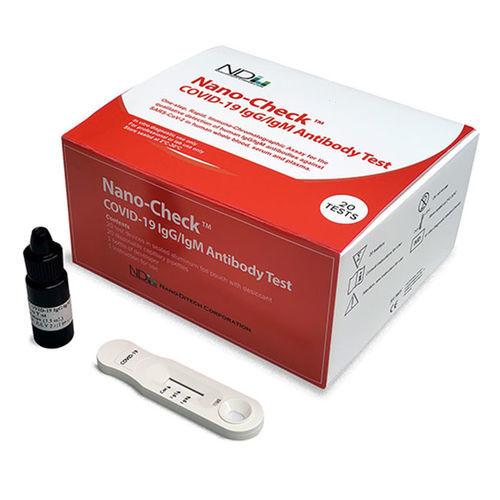
- COVID-19 rapid test
- Nano-Ditech Corporation
COVID-19 rapid test Nano-Check™IgGIgMSARS-COV-2
Add to favorites
Compare this product
Characteristics
- Applications
- COVID-19
- Tested parameter
- IgG, IgM
- Micro-organism
- SARS-COV-2
- Sample type
- serum, plasma, whole blood, clinical, laboratory
- Analysis mode
- CLIA
- Format
- strip
Description
Nano-CheckTM COVID-19 IgG/IgM Antibody Test is a rapid immunoassay for the qualitative detection of human IgG/IgM antibodies against SARS-CoV-2 in human whole blood, plasma and serum sample as an aid in the clinical diagnosis of patients with suspected SARS-CoV-2 infection. For prescription use only.
Test Principle
The membrane strip contains two test lines and one control line.
Antibody against human IgM and antibody against human IgG for test lines, goat anti-chicken IgY antibody for the control line.
A dye pad is placed at the end of the membrane containing gold colloidal particle coupled with recombinant SARS-CoV-2 antigen and gold colloidal particle coupled with chicken IgY
Nano-CheckTM COVID-19 IgG/IgM Antibody Test is a rapid immunoassay for the qualitative detection of human IgG/IgM antibodies to SARS-CoV-2 in human whole blood (venipuncture), plasma (Heparin/EDTA) and serum sample collected in CLIA certified laboratory or by healthcare workers at the point care.
The Nano-CheckTM COVID-19 IgG/IgM Antibody Test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection.
Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Results must be combined with clinical observations, patient history, and epidemiological information.
False positive results may occur due to cross-reactivity from pre-existing antibodies or other possible causes.
Catalogs
Related Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Rapid virus test
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Cassette assay kit
- Lateral flow test kit
- Rapid respiratory infection test
- Urine rapid diagnostic test
- COVID-19 rapid diagnostic test
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.