
- Company
- Products
- Catalogs
- News & Trends
- Exhibitions
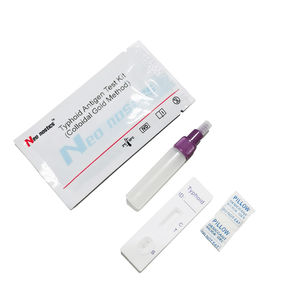
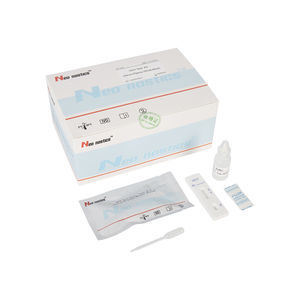
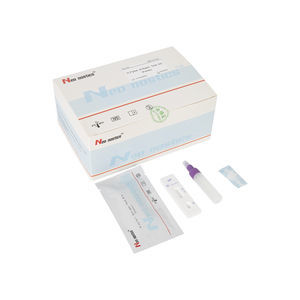
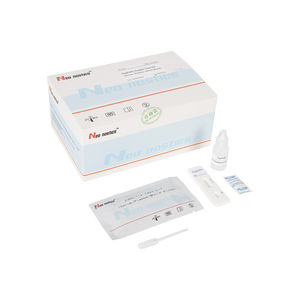
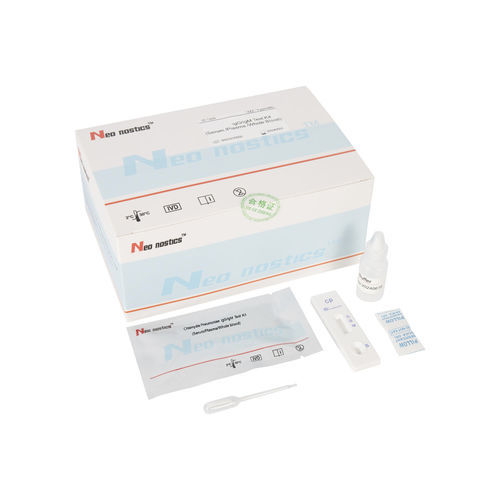
Chlamydia test kit CT00120 for antibodiesChlamydophila pneumoniaeserum
Add to favorites
Compare this product
Characteristics
- Applications
- Chlamydia
- Tested parameter
- for antibodies
- Micro-organism
- Chlamydophila pneumoniae
- Sample type
- serum, plasma, whole blood
- Analysis mode
- immunoassay
- Format
- cassette
- Other characteristics
- self-test
- Result display time
15 min
Description
This kit is used for in vitro qualitative detection of Chlamydia pneumoniae IgM/IgG antibodies in human venous plasma / serum or whole blood, samples .
Introduction
Chlamydia pneumoniae(CP) is a microorganism that lies between bacteria and viruses, belonging to the Chlamydia genus of the Chlamydia family. The pathogen is mainly transmitted through the respiratory tract and can cause respiratory infectious diseases, such as bronchitis, pneumonia, etc. In clinical practice, serological tests can generally be used to determine whether one is infected with Chlamydia pneumoniae. If the patient's blood routine test results show normal or low white blood cell count and an increase in lymphocyte proportion, it can be preliminarily diagnosed as Chlamydia pneumoniae infection. IgG antibodies are a type of immunoglobulin produced against Chlamydia pneumoniae. When the body is infected with Chlamydia pneumoniae, specific antibodies are produced in the body, resulting in IgG antibody positivity. Therefore, a positive IgG antibody and a negative IgM antibody for Chlamydia pneumoniae indicate that the patient has previously been infected with Chlamydia pneumoniae and has entered the recovery period.
Product Features
Easy handling, no instrument required.
3. Results are clearly visible and reliable.
4. High Accuracy.
5. Room temperature storage.
Test Procedure
1.Bring all materials, including specimen and test device, recover to 15-25ºC before running the assay.
2.Remove the test device from the foil pouch and place it horizontally.
3.Using the disposable dropper, place 1 drop of sample into sample hole "S" of the test device.
Catalogs
No catalogs are available for this product.
See all of Neo-nostics‘s catalogsRelated Searches
- Assay kit
- Blood assay kit
- Serum assay kit
- Immunoassay assay kit
- Plasma assay kit
- Infectious disease detection kit
- Blood rapid diagnostic test
- Rapid lateral flow test
- Immunoassay rapid diagnostic test
- Cassette rapid diagnostic test
- Virus rapid diagnostic test
- Respiratory infection test kit
- Whole blood detection kit
- Serum rapid diagnostic test
- Plasma rapid diagnostic test
- Infectious disease rapid diagnostic test
- Whole blood rapid diagnostic test
- Cassette assay kit
- Lateral flow test kit
- COVID-19 detection kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.