- Laboratory
- Laboratory medicine
- Drug detection test kit
- Neogen Corporation Inc.

- Products
- Catalogs
- News & Trends
- Exhibitions
Test kit for forensic applications 102319drug detectionfor quality controlELISA
Add to favorites
Compare this product
Characteristics
- Application field
- for forensic applications, drug detection, for quality control
- Analysis mode
- ELISA
- Result display time
75 min
- Sample volume
0.02 ml
(0.00068 US fl oz)
Description
Highly sensitive
Multiple matrices
Large menu of tests available
Neogen’s Levallorphan ELISA (Enzyme-Linked ImmunoSorbent Assay) kit is a qualitative one-step kit designed for use as a screening test for the detection of Levallorphan. The kit was designed for screening purposes and is intended for forensic use only.
Specifications
Result Type - Qualitative
Microplate Size - 96 well microplate
Intended Use - For the determination of trace quantities of Levallorphan and/or other metabolites in human urine, blood or oral fluid. Contact a Neogen representative for information on other matrices.
Sensitivity
The term I-50 is used to define the sensitivity of the test. This number is derived from a standard curve generated with the drug in EIA Buffer. The drug concentration that shows 50% less color activity than the zero standard is considered to be the I-50.
Drug Panel - Narcotic Analgesics
Storage Conditions - Test kit 2-8°; store controls -20°C
Wavelength -
650 nm with Red Stop Solution
450 nm with acid stop
Package Weight - 0.70 lb
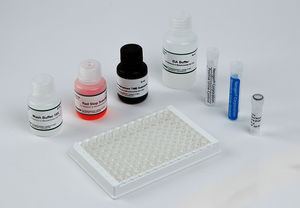
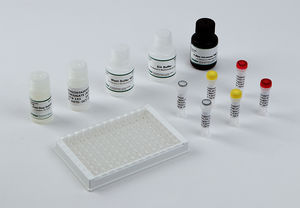
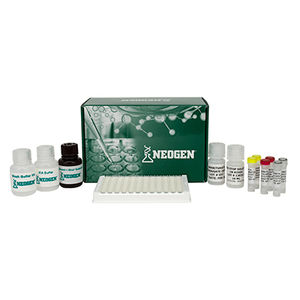
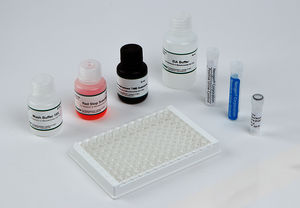
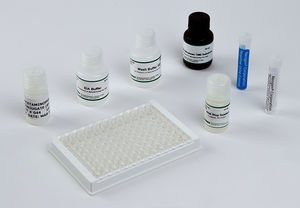
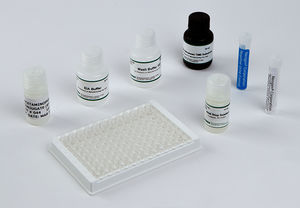
Materials Provided
EIA Buffer
Wash Buffer Concentrate (10X)
K-Blue Substrate
Drug-Enzyme Conjugate
Antibody Coated Plate
Red Stop Solution
Qualitative QC Positive Control
Qualitative QC Negative Control
Materials Required But Not Provided
Deionized water.
Precision pipettes that range from 10 μL - 1000 μL and disposable tips.
Graduated cylinder to dilute and mix wash buffer.
Plate cover or plastic film to cover plate during incubation.
Clean glassware (i.e. test tubes) to dilute samples.
Microplate reader with 650 nm filter.
Cut-off calibrator
Optional Materials
1N HCI if Red Stop Solution is not used
Microplate shaker
Related Searches
- Assay kit
- Solution reagent kit
- Blood assay kit
- Immunoassay assay kit
- Diagnostic reagent kit
- Enzyme reagent kit
- Reagent medium reagent kit
- Lateral flow test kit
- Clinical chemistry reagent
- ELISA assay kit
- Bacteria reagent kit
- Strip detection kit
- Laboratory detection kit
- Cell assay kit
- Urine assay kit
- Animal assay kit
- Genetic test kit
- Oral fluid test kit
- POC reader
- ELISA test reagent kit
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.