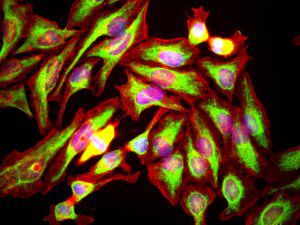
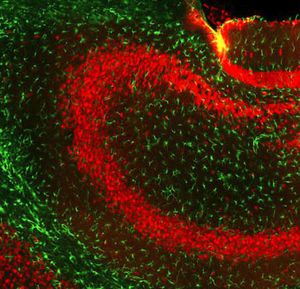
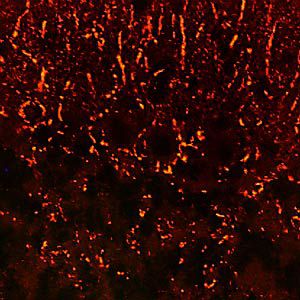
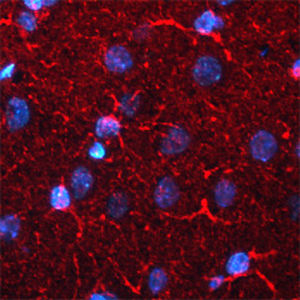
IgG reagent MO22196for Western blotfor immunofluorescencefor immunocytochemistry
Add to favorites
Compare this product
Characteristics
- Type
- IgG
- Applications
- for Western blot, for immunofluorescence, for immunocytochemistry
- Format
- liquid
- Tested parameter
- PEA15
- Origin
- mouse-based
- Storage temperature
Max.: 4 °C
(39 °F)Min.: -20 °C
(-4 °F)
Description
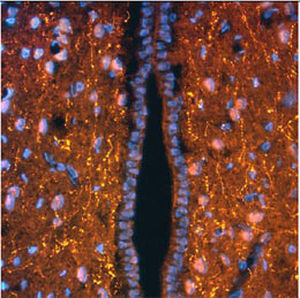
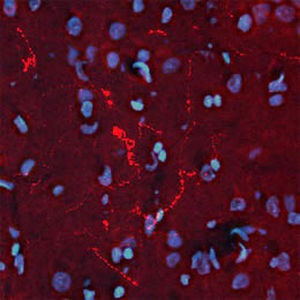
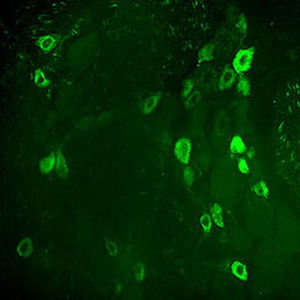
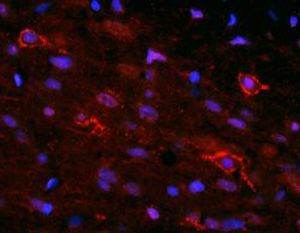
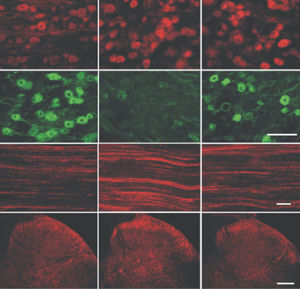
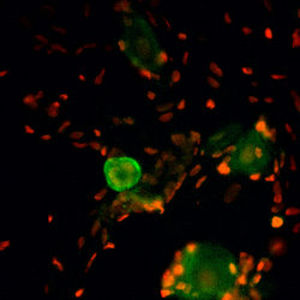
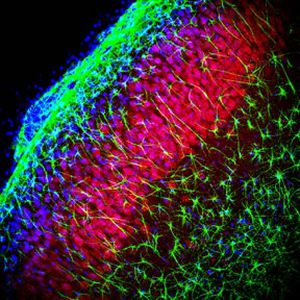
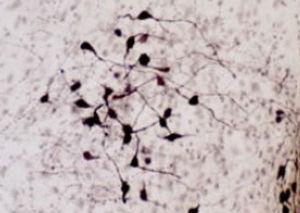
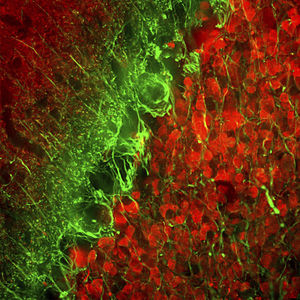
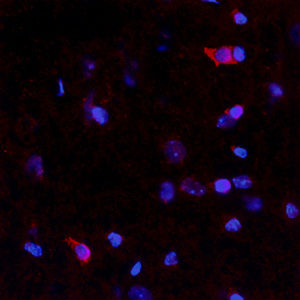
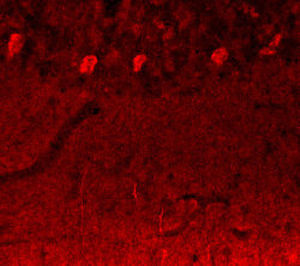
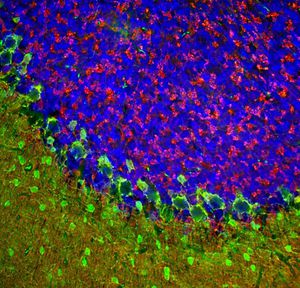
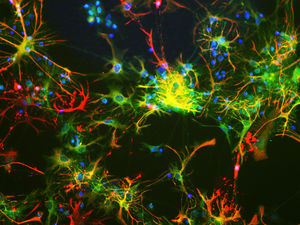
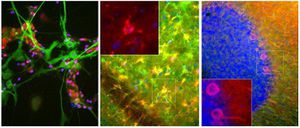
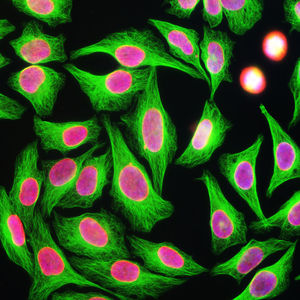
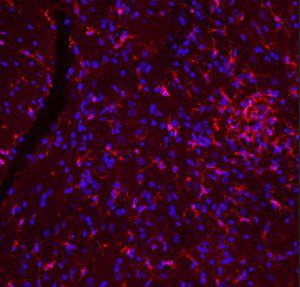
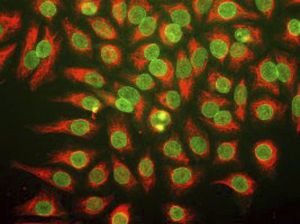
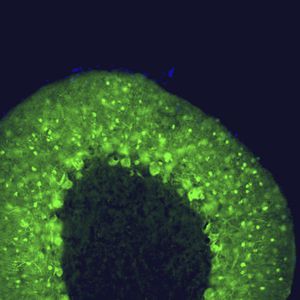
PEA-15 was originally isolated as a major low molecular weight of embryonic mouse striatal astrocytes grown in cell culture. Three spots on 2D gels with an apparent molecular weight of 15kDa and isoelectric point 5.1-5.3 were shown to be different forms of one protein. The protein was serine phosphorylated on one site by protein kinase C both in vivo and in vitro and the protein was named “phosphoprotein enriched in astrocytes of 15kDa”, hence PEA-15. Subsequent cloning and sequencing revealed a protein well conserved in sequence between mouse and human and which was heavily expressed in brain. Independently the same protein was found to be upregulated in fibroblasts and tissues of diabetic patients, and has hence named “protein enriched in diabetes” or PED. Immunocytochemical studies showed that the protein was heavily expressed in astrocytes and certain neurons in the CNS of mice, though it is expressed a lower levels ubiquitously. PEA-15 was shown to interact with extracellular signal regulated kinase and regulate the nuclear entry of this protein, and several other important interactions with other proteins involved in regulation of apoptosis, glucose metabolism and cell growth have been described. MCA-4D2 was made against a recombinant full length PEA-15 construct expressed in and purified from E. coli.
Catalogs
No catalogs are available for this product.
See all of Neuromics‘s catalogsRelated Searches
- Molecular biology reagent kit
- Research reagent kit
- Analysis software
- Protein reagent kit
- Immunology reagent
- Antibody
- Laboratory software
- Lyophilized reagent kit
- Serum reagent kit
- Immunohistochemistry reagent kit
- Enzyme reagent
- Monoclonal antibody reagent kit
- Scientific research reagent kit
- Western blot reagent kit
- Research software
- Immunofluorescence reagent kit
- Polyclonal antibody
- Cytokine reagent kit
- Growth factor reagent kit
- Mouse-based reagent
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.