IgG reagent MO22149for scientific researchfor Western blotimmunohistochemistry
Add to favorites
Compare this product
Characteristics
- Type
- IgG
- Applications
- for scientific research, for Western blot, immunohistochemistry, for immunofluorescence, for immunocytochemistry
- Format
- liquid
- Tested parameter
- parvalbumin
- Origin
- mouse-based
- Storage temperature
Min.: -20 °C
(-4 °F)Max.: 4 °C
(39 °F)
Description
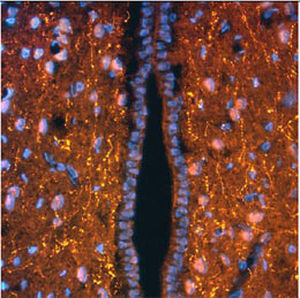
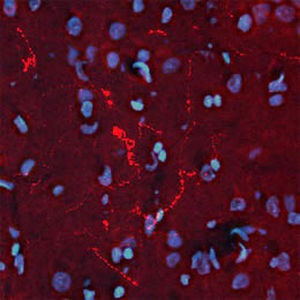
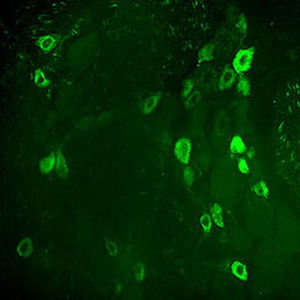
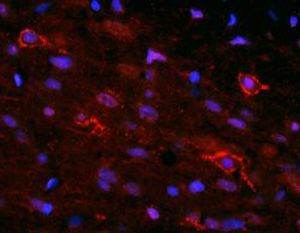
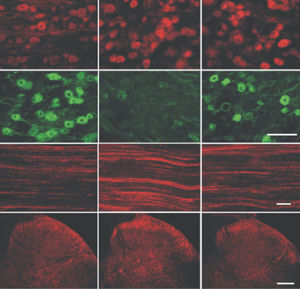
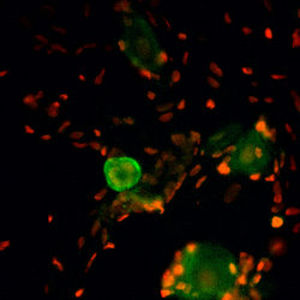
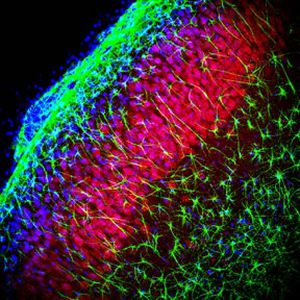
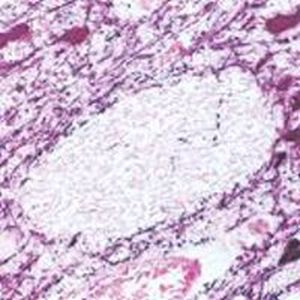
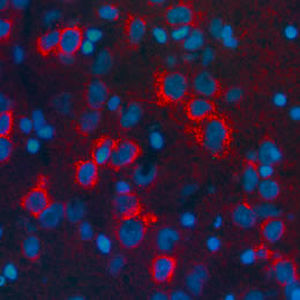
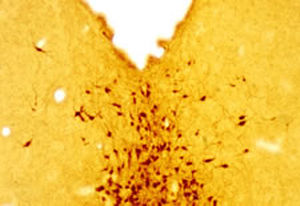
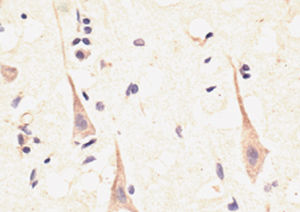
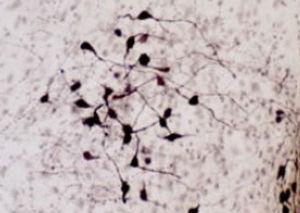
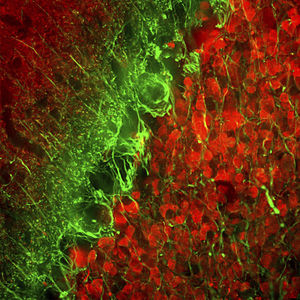
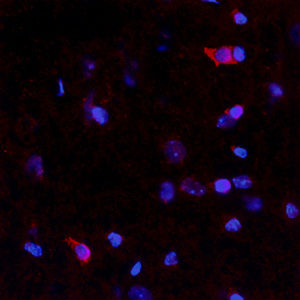
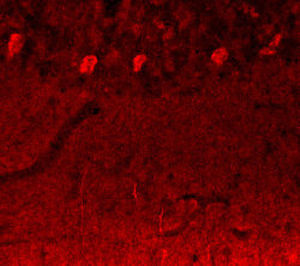
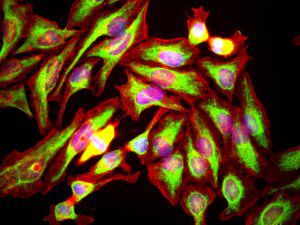
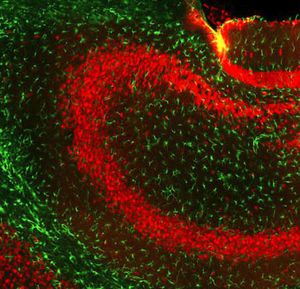
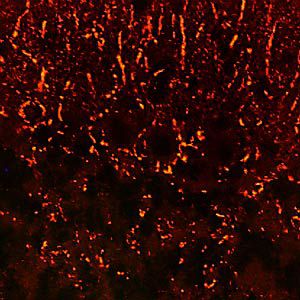
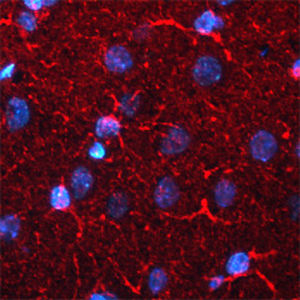
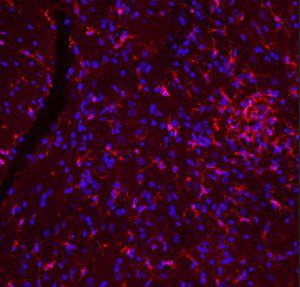
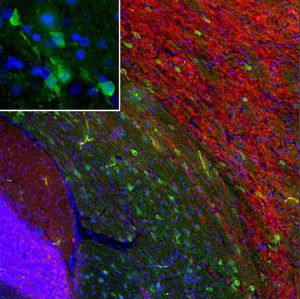
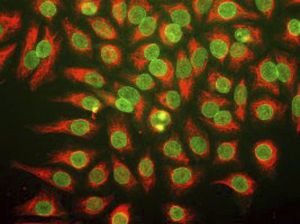
Parvalbumin is a cytoplasmic low molecular weight Ca2+ binding proteins with. It is the prototypic member of the very large family of proteins containing the "EF hand" Ca2+ binding motif. The nomenclature comes from the parvalbumin structure in which the fifth and sixth alpha helices, the E and F helices, form a V shape including acid amino acids which co-ordinate a single Ca2+. It turns out that close variants of this structure are found in many other Ca2+ binding proteins. Parvalbumin is expressed in fast-contracting muscles, where its levels are highest, as well as in the brain and some endocrine tissues. In brain, it is particularly concentrated in Purkinje cells and interneurons in the molecular layer of the cerebellum, but is also found in many GABAergic interneurons in the cortex. These GABAergic interneurons in most cases express only one of three Ca2+ binding proteins, namely parvalbumin, calretinin, or calbindin. As a result, these important inhibitory interneurons can be identified and subclassified based on their content of these three proteins. Each type of neuron as defined in this fashion has particular electrophysiological and functional properties. For example, calbindin positive interneurons are not fast-spiking as are parvalbumin expressing interneurons.
Catalogs
No catalogs are available for this product.
See all of Neuromics‘s catalogsRelated Searches
- Molecular biology reagent kit
- Research reagent kit
- Analysis software
- Protein reagent kit
- Immunology reagent
- Antibody
- Laboratory software
- Lyophilized reagent kit
- Serum reagent kit
- Immunohistochemistry reagent kit
- Enzyme reagent
- Monoclonal antibody reagent kit
- Western blot reagent kit
- Scientific research reagent kit
- Research software
- Immunofluorescence reagent kit
- Polyclonal antibody
- Cytokine reagent kit
- Growth factor reagent kit
- Mouse-based reagent
*Prices are pre-tax. They exclude delivery charges and customs duties and do not include additional charges for installation or activation options. Prices are indicative only and may vary by country, with changes to the cost of raw materials and exchange rates.